Abstract
Donor lymphocyte infusions (DLI) can produce lasting remissions in patients with relapsed chronic myeloid leukemia (CML), but are less effective in non-CML diseases. Chemotherapy-induced lymphodepletion prior to DLI, achieved with cyclophosphamide (Cy) and fludarabine (Flu), has been shown to enhance activation of donor lymphocytes and cause significantly more acute graft-versus-host disease (GVHD) than DLI alone. To safely balance the toxic versus beneficial effects of activated donor lymphocytes, we combined lymphodepleting chemotherapy with the infusion of donor T cells engineered to carry a suicide gene to treat patients with aggressive hematologic malignancies.
Donor T cells were transduced with a retroviral vector expressing the Herpes simplex thymidine kinase (TK) suicide gene and the human Thy-1 selection marker and expanded in culture for 15 to 19 days prior to their infusion. In this phase I/II trial, the safety and efficacy of Cy/Flu/DLI-TK was studied between February and December 2011 in 10 adults with relapsed multiple myeloma (n=5) or myelodysplasic syndrome/acute leukemia (n=5). One had previously failed to respond to at least one standard DLI while others had not received any DLI before inclusion. All were free of immunosuppressive therapy at inclusion. Patients were infused with a mean cell-dose of 5 (range: 1-10) x106 CD3+ cells/kg of which 98% were TK+ cells (range: 97%-99%). DLI products contained a mean ratio of 0.6% (range: 0.1-1.6) CD4+FoxP3+Helios+ regulatory T-cells. This trial was registered at www.clinicaltrials.gov as NCT 01086735.
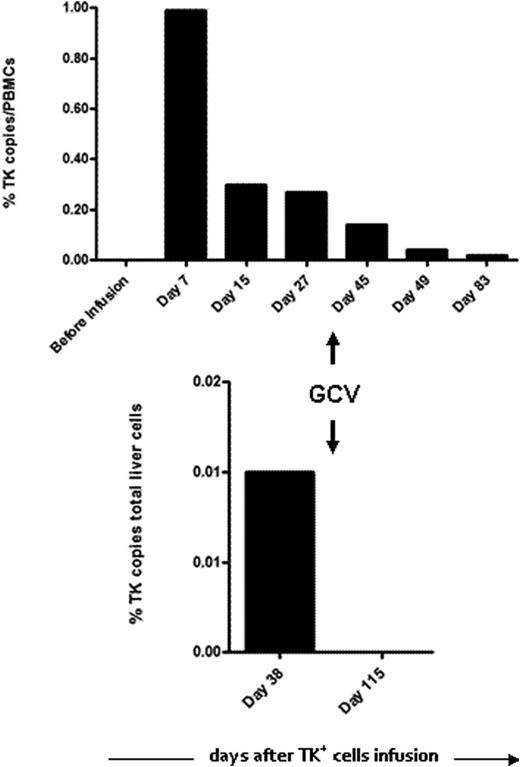
The Cy/Flu regimen was profoundly lymphodepleting and led to a mean 3-day period of neutropenia below 500 PMN/µL. Three patients developed acute GVHD following TK+ cell infusion. One patient had a grade I cutaneous GVHD resolving with local steroids. In another patient with grade III cutaneous and digestive involvement, intra-venous administration of ganciclovir (GCV) led to the complete and lasting resolution of symptoms correlated with clearance of TK+ cells in peripheral blood. A third patient had a grade III liver GVHD. Due to only partially controlled leukemia at the time of DLI, GCV treatment was postponed for one week after GVHD onset in order to support a putative GVL effect. Treatment with GCV led to the rapid disappearance of TK+ cells in peripheral blood and liver (see Figure) but without clinical improvement after 2 weeks, so that an additional immunosuppressive therapy should be instituted. Six patients, including one of the 2 treated with GCV, experienced relapse/progression of their malignancy and received additional anti-cancer therapy. With a median follow-up of 22 months after Cy/Flu/DLI-TK, 6 patients are alive, 5 of them being disease-free. In patients not treated with GCV, TK+ cells could still be detected in peripheral blood till at least 12 months after their infusion.
Lymphodepletion followed by suicide-gene-transduced T-cell infusion may help to safely enhance the immune activity of DLI, which could be further improved by regulatory T-cell depletion (Maury et al, Science Translat Medicine 2010).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal