Abstract
The Wilms' tumor gene (WT1), originally discovered as a tumor suppressor has been proven to have an oncogenic role in leukemia and several other cancers. WT1 mRNA expression levels in peripheral blood (PBWT1) has been reported as a useful marker for the risk evaluation of myelodysplastic syndrome (MDS). In the era of hypomethylating agents, the significance of PBWT1 on MDS prognosis is still unknown. This study aimed to clarify the impact of pre-treatment PBWT1 levels on overall response (OR) and overall survival (OS) in MDS patients treated with azacitidine (AZA).
We retrospectively analyzed all patients from March 2011 to March 2013 with World Health Organization 2008 defined MDS, CMML or AML with 20–30% bone marrow blasts who received AZA treatment in our department for at least one cycle (37.5–75.0 mg/m2/day during 7 days, every 28 days). Patients' peripheral blood specimens were collected before AZA initiation, mRNA was extracted from leukocytes using the RNeasy Mini-Kit (Qiagen, Valencia, CA), and the amount containing WT1 mRNA was measured using a WT1 mRNA Assay Kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). Hematologic response was evaluated according to International Working Group 2006. OR was defined as a best overall response of complete remission (CR), partial remission, marrow CR, or hematologic improvement. Univariate analyses for OR were carried out using Fisher's exact test. Factors associated with at least borderline significance (p < 0.10) were subjected to a multivariate analysis, using logistic regression model. OS was estimated according to the Kaplan-Meier method. Multivariate analysis was performed with proportional hazard Cox model, including all variables with p < 0.10 in univariate analyses.
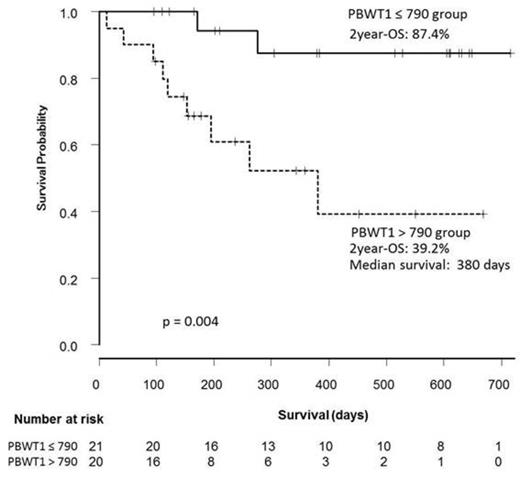
Of 55 patients enrolled, pre-treatment PBWT1 levels were available in 41 patients and the median level was 790 copies/µg RNA (range, less than 50–310000). Baseline characteristics according to PBWT1 levels (≤ 790 [lower group] [n = 21] and > 790 [higher group] [n = 20]) are summarized in Table 1. Median number of AZA treatment cycles was 4 (range, 1–18). Four patients (2 in higher group, and 2 in lower group) received allogeneic stem cell transplantation (alloSCT) after AZA treatment. OR rates were significantly lower in PBWT1 higher group than lower group (30.0 vs 71.4%, p = 0.03). In univariate analysis, other significant risk factors or with borderline significance for OR were higher serum ferritin levels (> 1000 ng/ml) and RBC transfusion dependency ≥ 4 units/8 weeks. In multivariate analysis, higher PBWT1 levels independently predicted reduced likelihood of OR (odds ratio = 0.212, 95% CI 0.01-0.95, p = 0.02). OS was significantly inferior in PBWT1 higher group as shown in Figure 1. In univariate analysis, other significant factor was Revised International Prognostic Scoring System (IPSS-R) risk groups (high risk defined as IPSS-R high or higher, and low risk defined as IPSS-R intermediate or lower). In multivariate analysis, higher PBWT1 levels (hazard ratio [HR] = 9.75, 95% CI 1.22-77.58, p = 0.03) and IPSS-R high risk (HR=7.04, 95% CI 1.43-34.48, p = 0.02) were independent predictors for OS.
Patient baseline characteristics according to pre-treatment PBWT1 levels
| . | PBWT1(copies/µg RNA) . | . | |
|---|---|---|---|
| . | ≤ 790 (n=21) . | >790 (n=20) . | p value . |
| Median age, years | 71.0 | 70.5 | |
| WHO 2008 classification, % | 0.202 | ||
| RARS | 4.8 | 0.0 | |
| RCMD | 19.0 | 10.0 | |
| RAEB-1 | 23.8 | 40.0 | |
| RAEB-2 | 38.1 | 40.0 | |
| CMML-1 | 14.3 | 0.0 | |
| AML | 0.0 | 10.0 | |
| IPSS-R risk group, % | 0.003 | ||
| Very low | 0.0 | 0.0 | |
| Low | 28.6 | 0.0 | |
| Intermediate | 33.3 | 30.0 | |
| High | 33.3 | 25.0 | |
| Very high | 4.8 | 45.0 | |
| Absolute neutrophil count, mean ± SD, 103/µl | 2.0 ± 2.6 | 1.4 ± 1.2 | 0.392 |
| Platelets, mean ± SD, 104/µl | 15.1 ± 25.6 | 10.8 ± 18.1 | 0.545 |
| Hb level, mean ± SD, g/dl | 9.5 ± 2.3 | 7.7 ± 1.5 | 0.008 |
| Ferritin level, mean ± SD, ng/ml | 531.1 ± 902.1 | 741.0 ± 1008.1 | 0.503 |
| Marrow blast, % | 0.111 | ||
| < 5 % | 52.3 | 25.0 | |
| ≥ 5 % | 47.6 | 75.0 | |
| RBC transfusion dependency, % | 0.326 | ||
| Yes | 23.8 | 40.0 | |
| No | 76.2 | 60.0 | |
| . | PBWT1(copies/µg RNA) . | . | |
|---|---|---|---|
| . | ≤ 790 (n=21) . | >790 (n=20) . | p value . |
| Median age, years | 71.0 | 70.5 | |
| WHO 2008 classification, % | 0.202 | ||
| RARS | 4.8 | 0.0 | |
| RCMD | 19.0 | 10.0 | |
| RAEB-1 | 23.8 | 40.0 | |
| RAEB-2 | 38.1 | 40.0 | |
| CMML-1 | 14.3 | 0.0 | |
| AML | 0.0 | 10.0 | |
| IPSS-R risk group, % | 0.003 | ||
| Very low | 0.0 | 0.0 | |
| Low | 28.6 | 0.0 | |
| Intermediate | 33.3 | 30.0 | |
| High | 33.3 | 25.0 | |
| Very high | 4.8 | 45.0 | |
| Absolute neutrophil count, mean ± SD, 103/µl | 2.0 ± 2.6 | 1.4 ± 1.2 | 0.392 |
| Platelets, mean ± SD, 104/µl | 15.1 ± 25.6 | 10.8 ± 18.1 | 0.545 |
| Hb level, mean ± SD, g/dl | 9.5 ± 2.3 | 7.7 ± 1.5 | 0.008 |
| Ferritin level, mean ± SD, ng/ml | 531.1 ± 902.1 | 741.0 ± 1008.1 | 0.503 |
| Marrow blast, % | 0.111 | ||
| < 5 % | 52.3 | 25.0 | |
| ≥ 5 % | 47.6 | 75.0 | |
| RBC transfusion dependency, % | 0.326 | ||
| Yes | 23.8 | 40.0 | |
| No | 76.2 | 60.0 | |
Our results suggest that PBWT1 can predict both response and survival of MDS patients treated with AZA. Although salvage therapy including alloSCT can affect the survival, poor survival might result from inferior response rates in PBWT1 high patients. In MDS with high PBWT1, restoration of epigenetically silenced tumor suppressor genes with AZA might not induce apoptosis. We propose that alternative therapeutic strategies should be sought in MDS patients with high PBWT1 levels.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal