Abstract
Expression of CD25 on blasts of AML patients has been shown to hold independent prognostic value for both survival and response to induction therapy. Patients with MDS-related AML have generally higher CD25 expression from de novo AML patients, but though there is paucity of a serviceable biomarker of outcome in MDS patients treated with azacytidine, the prognostic value of CD25 has not yet been investigated.
Bone marrow samples of 61 patients with intermediate-2/high risk IPSS, high/very high WPSS and low blast count AML were obtained before and 15 days (D15) after the initiation of treatment. All patients received azacytidine in a non clinical trial setting at an initial dose of 75mg/m2 SC for 7 days on 28-day cycles. CD25 expression was assessed by 4-color flow cytometry on total CD34+ blasts, committed progenitors (Lin-CD38+CD34+) and leukemic stem cells (LSC, Lin-CD38-CD34+). Positivity was defined as a CD25 expression of ≥ 20%. Statistical comparisons were done by ÷2, one-way ANOVA and paired or unpaired t-test as appropriate, and survival with Kaplan-Meier analysis and log-rank test. Overall survival (OS) was defined as the time from azacytidine initiation to death from any cause and event-free survival (EFS) as the time from diagnosis to disease progression, relapse or death. Multivariate survival analysis was based on Cox’s proportional hazards model using a backward stepwise selection procedure with entry and removal criteria of p=0.05 and p=0.10, respectively.
Patient characteristics. N/A: not applicable/not available.
| . | CD25- (n=36) . | CD25+ (n=25) . | p-value . |
|---|---|---|---|
| Age | 72,5 (53,4-83.5) | 72,9 (52-81.7) | 0.2 |
| >65 | 31(86%) | 17(68%) | |
| <65 | 5(14%) | 8(32%) | |
| Sex | 0.027 | ||
| Male | 20(55%) | 21(84%) | |
| Female | 16(45%) | 4(16%) | |
| WHO classification | 0.28 | ||
| RCMD | 2(5%) | 0(0%) | |
| RAEB-I | 0(0%) | 1(4%) | |
| RAEB-II | 17(47%) | 13(52%) | |
| CMML-II | 6(16%) | 7(28%) | |
| Low blast count - sAML | 11(30%) | 4(16%) | |
| Baseline blood counts | |||
| Hemoglobin (g/dl) | 8,6(6.1-10.6) | 8,8(6.8-11.5) | 0.78 |
| ANC(x 109/L) | 1,1(0.04-13.4) | 2,8(0.08-23) | 0.07 |
| Platelets (x 109/L) | 66(9-383) | 50(11-181) | 0.42 |
| IPSS | 0.6 | ||
| Intermediate-2 | 17(47%) | 9(36%) | |
| High | 8 (22%) | 6(24%) | |
| N/A | 11(31%) | 10(40%) | |
| WPSS | 0.22 | ||
| High | 15(42%) | 8(32%) | |
| Very high | 6(16%) | 7(28%) | |
| N/A | 15(42%) | 10(40%) | |
| IPSS-R | 0.8 | ||
| Intermediate | 3(8%) | 1(4%) | |
| High | 14(39%) | 8(32%) | |
| Very high | 17(48%) | 12(48%) | |
| N/A | 2(5%) | 6(24%) | |
| IPSS-R Cytogenetic risk | 0.72 | ||
| Good | 18(50%) | 10(40%) | |
| Intermediate | 8(22%) | 6(24%) | |
| Poor | 5(14%) | 6(24%) | |
| Very poor | 4(11%) | 2(8%) | |
| N/A | 1(3%) | 1(4%) | |
| PB blasts | 0.5 | ||
| Present | 19(53%) | 13(52%) | |
| Absent | 14(39%) | 11(44%) | |
| N/A | 3(8%) | 1(4%) | |
| BM blasts | 0.2 | ||
| >15% | 18(50%) | 9(36%) | |
| ≤15% | 18(50%) | 16(54%) | |
| Transfusions ≥ 4 per month | 0.48 | ||
| Yes | 23(4%) | 17(4%) | |
| No | 13(4%) | 8(4%) | |
| Response | 0.4 | ||
| CR & PR | 12(33%) | 4(16%) | |
| Hematologic improvement | 5(14%) | 4(16%) | |
| Stable disease | 8(22%) | 5(20%) | |
| Failure | 11(31%) | 12(48%) |
| . | CD25- (n=36) . | CD25+ (n=25) . | p-value . |
|---|---|---|---|
| Age | 72,5 (53,4-83.5) | 72,9 (52-81.7) | 0.2 |
| >65 | 31(86%) | 17(68%) | |
| <65 | 5(14%) | 8(32%) | |
| Sex | 0.027 | ||
| Male | 20(55%) | 21(84%) | |
| Female | 16(45%) | 4(16%) | |
| WHO classification | 0.28 | ||
| RCMD | 2(5%) | 0(0%) | |
| RAEB-I | 0(0%) | 1(4%) | |
| RAEB-II | 17(47%) | 13(52%) | |
| CMML-II | 6(16%) | 7(28%) | |
| Low blast count - sAML | 11(30%) | 4(16%) | |
| Baseline blood counts | |||
| Hemoglobin (g/dl) | 8,6(6.1-10.6) | 8,8(6.8-11.5) | 0.78 |
| ANC(x 109/L) | 1,1(0.04-13.4) | 2,8(0.08-23) | 0.07 |
| Platelets (x 109/L) | 66(9-383) | 50(11-181) | 0.42 |
| IPSS | 0.6 | ||
| Intermediate-2 | 17(47%) | 9(36%) | |
| High | 8 (22%) | 6(24%) | |
| N/A | 11(31%) | 10(40%) | |
| WPSS | 0.22 | ||
| High | 15(42%) | 8(32%) | |
| Very high | 6(16%) | 7(28%) | |
| N/A | 15(42%) | 10(40%) | |
| IPSS-R | 0.8 | ||
| Intermediate | 3(8%) | 1(4%) | |
| High | 14(39%) | 8(32%) | |
| Very high | 17(48%) | 12(48%) | |
| N/A | 2(5%) | 6(24%) | |
| IPSS-R Cytogenetic risk | 0.72 | ||
| Good | 18(50%) | 10(40%) | |
| Intermediate | 8(22%) | 6(24%) | |
| Poor | 5(14%) | 6(24%) | |
| Very poor | 4(11%) | 2(8%) | |
| N/A | 1(3%) | 1(4%) | |
| PB blasts | 0.5 | ||
| Present | 19(53%) | 13(52%) | |
| Absent | 14(39%) | 11(44%) | |
| N/A | 3(8%) | 1(4%) | |
| BM blasts | 0.2 | ||
| >15% | 18(50%) | 9(36%) | |
| ≤15% | 18(50%) | 16(54%) | |
| Transfusions ≥ 4 per month | 0.48 | ||
| Yes | 23(4%) | 17(4%) | |
| No | 13(4%) | 8(4%) | |
| Response | 0.4 | ||
| CR & PR | 12(33%) | 4(16%) | |
| Hematologic improvement | 5(14%) | 4(16%) | |
| Stable disease | 8(22%) | 5(20%) | |
| Failure | 11(31%) | 12(48%) |
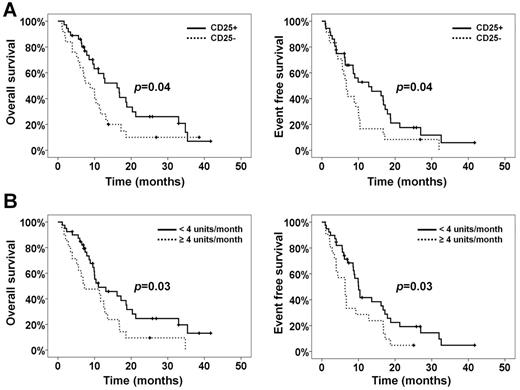
(A) OS and EFS according to CD25 positivity status. (B) OS and EFS according to transfusion requirements.
(A) OS and EFS according to CD25 positivity status. (B) OS and EFS according to transfusion requirements.
Collectively, our findings reveal an independent prognostic role for CD25 in MDS patients treated with azacytidine. In addition, the differential expression and epigenetic modulation of CD25 in the LSC compartment support the investigation of therapeutic strategies using monoclonal antibody targeting combined with epigenetic agents.
Kotsianidis:Genesis Hellas: Honoraria, Research Funding. Spanoudakis:Genesis Hellas: Honoraria. Tsatalas:Genesis Hellas: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal