Abstract

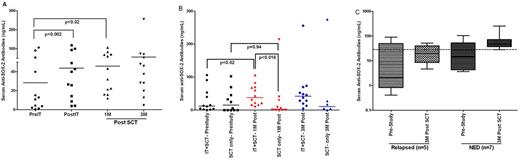
A phase Ib trial was conducted in standard and high risk multiple myeloma (MM) patients to determine whether infusions of anti-CD3 x anti-CD20 bispecific antibody (CD20Bi) armed activated T cells (aATC) prior to autologous stem cell transplant (ASCT) were safe and would induce anti-myeloma (anti-MM) immunity that could be detected after ASCT. We hypothesized that targeting CD20+/CD138- myeloma clonogenic cells with aATC would release “MM antigens” including anti-Sry-HMG-box 2 (SOX-2). Engagement of target cells by aATC also induces release of Th1 cytokines by aATC creating an environment conducive for in situ immunization against MM antigens. Twelve patients received two infusions of 1010 one week apart and within 4 weeks prior to ASCT. Four patients received a 10 x 109 aATC booster infusion between 6 and 18 months after ASCT. There were significant increases in the fraction of patients who developed IFN-g EliSpots directed at CD20+ Daudi lymphoma cells (p<0.06), CD20 negative RPMI 8226 multiple myeloma cells (p<0.02), and K562 natural killer cell targets (p<0.01) from 6 to 12 months after ASCT. Specific cytotoxicity directed at Daudi (p<0.03), K562 (p<0.009), and RPMI 8226 (p<0.02) targets increased significantly after aATC infusions and post ASCT compared to pre infusion baseline cytotoxicity. SOX-2 IgG antibody levels increased above baseline after aATC infusions (p<0.002) and post ASCT (p<0.02) (Fig. 1A). There was no significant difference observed between pre and 1 month post ASCT in anti-SOX-2 levels or between pre and 3 month post ASCT serum samples from control ASCT patients who did not receive IT (Fig. 1B). In order to determine whether there was an association between the level of anti-SOX-2 antibodies and clinical outcome, anti-SOX-2 antibody levels were plotted in patients who progressed and those who did not progress. The box plots show the clear separation between two groups. The median anti-SOX-2 antibody levels at 3 months after ASCT in patients who relapsed was 19.7 ng/ml (25-75%, 8.6 - 40.3) and in patients who remained in remission was 48.1 ng/ml (37.5 - 73.3), these data suggest that anti-SOX-2 levels may predict clinical outcomes (Fig. 1C). There were no dose-limiting toxicities. In summary, aATC infusions pre ASCT induced anti-myeloma IFN-g EliSpot and anti-SOX-2 IgG responses that could be detected at 6-12 months after ASCT and these responses were boosted in 4 of 4 patients after ASCT. Data suggest that infusions of targeted T cells given prior to ASCT could induce cellular and humoral anti-myeloma immunity that could be transferred, detected, and boosted after ASCT. These results have implications for the design of immunotherapy in combination with ASCT.
Disclosures:
Lum:Transtarget Inc: Equity Ownership, In situ Immunization Patent, In situ Immunization Patent Patents & Royalties.
Author notes
*
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal