Abstract
Cytogenetic normal acute myeloid leukemia (CN-AML) represents 40-50% of new AML patients, where molecular characterisation of this subgroup is paramount in the therapeutic algorithm. Flt3, NPM1, cKIT and CEBPA molecular mutations are clinically established, whilst mutations in epigenetic modifiers such as, Tet2, IDH1, IDH2 and DNMT3A are rapidly evolving into clinical relevance. However, not all centres have access to these state-of-the-art molecular assays, a good proportion of CN-AML patients will either not express or have contrasting predictive molecular phenotypes and the positive predictive value for especially, “favourable” (eg. NPM1 mutation) molecular markers are modest. In situ analysis of T cell immunity in colorectal cancer has been reported to be a better predictor of survival than the histopathology TNM score (J.Galon et al. Science 2006). Also, in NPM1 mutated CN-AML, T cell responses have been documented both at diagnosis (J. Greiner et al., Blood 2012) and post donor lymphocyte infusion therapy at relapse (S. Hofmann et al., JCO 2012). Given the molecular heterogeneity and disparity in clinical outcome of CN-AML, and evidence of T cell activity in both solid and blood cancers, we hypothesised that the adaptive immune state may be a novel prognostic marker in the CN-AML subgroup.
A retrospective analysis was performed on available diagnostic trephine biopsies from CN-AML patients over 2 non-consecutive years in 2006-2007 and 2010-2011. Primary endpoint was overall survival. Trephine sections were stained for CD3, CD4, CD8 and Granzyme B. Accurate quantification of these cells was determined using automated imaging thresholding techniques developed by Monash Micro Imaging platform that utilised ImageJ (v1.46s) macro programmes. Survival was estimated using the Kaplan-Meier method and patient categories based on percentage positivity for each immunostain were compared using the log-rank test.
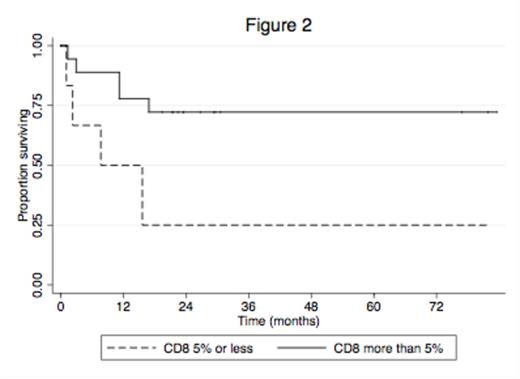
26 evaluable patients (15 female; 11 male; Age range 23-90 years) were identified. Median follow up was 26 months. 7 patients were in 1st complete remission (CR1); 4 patients were in 2nd CR; 2 patients had stable disease; 9 patients were deceased; 4 patients were lost to follow up. 3/18 patients tested were positive for FLT3 ITD (2 in CR; 1 deceased). 5/14 patients tested were positive for NPM1, of which 1/5 was also FLT3-ITD positive (2 in CR; 3 deceased). Prominent non-specific background staining prevented CD4 analyses. The mean staining scores for CD3 and CD8 were 16% and 11%, respectively. Six patients (23%) in each of the CD3 and CD8 groups scored =<5% staining, where 5 of these respective 6 patients scored =<5% for both CD3 and CD8. Analyses for both CD3 and CD8 stains at >5% vs =<5% showed a trend for increased survival with estimated 3-year survivals of 71% vs. 33%, p=0.054 and 72% vs. 25%, p=0.027, respectively (Figures 1 and 2). The median for Granzyme B stain was 1% and when analysed at >1% vs =<1%, also showed improved survival, although this was not statistically significant (69% vs. 53%, p=0.317).
Our initial evaluation of immunity in CN-AML patients suggests reduced T cell status at diagnosis may predict a poor prognosis for overall survival. Of note, there was not a vast difference in the age of patients with CD3 and/or CD8 stains =<5% vs >5% (mean age of 62 vs 54.5 years, respectively). Based on these preliminary results, we will further assess the prognostic value of CD3, CD8 and Granzyme B staining in correlation with molecular markers in a larger cohort of CN-AML patients.

No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal