Abstract
ITP is an autoimmune disorder characterized by isolated thrombocytopenia in the absence of other causes. Although the pathogenetic mechanisms may vary between cases, in common is the accelerated peripheral destruction of platelets due to a failure of self-tolerance and a complex interplay between autoantibodies, autoreactive T cells, antigen-presenting cells and the reticuloendothelial system. Conventional therapies such as glucocorticoids and rituximab target only one or some of these players in autoimmunity.
To demonstrate the safety and efficacy of a triple therapy approach to targeting all three major agents for autoimmunity in ITP: B-cells, T-cells and antigen-presenting cells. Simultaneous suppression of these three cell lines may benefit responders with prolonged relapse free survival without the burden of significant additional toxicity.
A total of 26 patients with ITP were enrolled into two separate sites with three separate triple therapy protocols (TT4, R1 and R2). Eligibility criteria, definitions and toxicity are as per IWG criteria and CTCAE 4.02.
Demographics and treatment schedules
| . | TT4 (n=11) . | R1 (n=6) . | R2 (n=9) . | All triple therapies (n=26) . |
|---|---|---|---|---|
| Median age, years [range] | 49 [19-85] | 38.5 [22-54] | 48 [14-79] | 47 [14-85] |
| Female, % | 45 | 83 | 56 | 58 |
| Median lines of therapy | 2 [0-7] | 4 [2-6] | 3 [2-7] | 3 [0-7] |
| Splenectomy (%) | 2 (18) | 1 (17) | 1 (11) | 4 (15) |
| Median from diagnosis, months | 125 [1-215] | 86 [1-223] | 26 [1-222] | 66 [1-223] |
| Treatment schedule | Dexamethasone 40mg PO D1-4; rituximab IV 100mg D7, 14, 21, 28; cyclosporine 2.5-3mg/kg/day BD PO D1-28 | methylprednisolone 1g IV D1-3; rituximab 500mg IV x4; mycophenolate mofetil 180mg BD PO x6 months | methylprednisolone 1g IV D1-3; rituximab 500mg IV x4; cyclosporine 100mg BD PO x6 months |
| . | TT4 (n=11) . | R1 (n=6) . | R2 (n=9) . | All triple therapies (n=26) . |
|---|---|---|---|---|
| Median age, years [range] | 49 [19-85] | 38.5 [22-54] | 48 [14-79] | 47 [14-85] |
| Female, % | 45 | 83 | 56 | 58 |
| Median lines of therapy | 2 [0-7] | 4 [2-6] | 3 [2-7] | 3 [0-7] |
| Splenectomy (%) | 2 (18) | 1 (17) | 1 (11) | 4 (15) |
| Median from diagnosis, months | 125 [1-215] | 86 [1-223] | 26 [1-222] | 66 [1-223] |
| Treatment schedule | Dexamethasone 40mg PO D1-4; rituximab IV 100mg D7, 14, 21, 28; cyclosporine 2.5-3mg/kg/day BD PO D1-28 | methylprednisolone 1g IV D1-3; rituximab 500mg IV x4; mycophenolate mofetil 180mg BD PO x6 months | methylprednisolone 1g IV D1-3; rituximab 500mg IV x4; cyclosporine 100mg BD PO x6 months |
25/26 patients completed treatment as planned. Three required salvage glucocorticoids/IVIG during the first four weeks of therapy. One patient additionally required splenectomy and completed triple therapy.
There was only one therapy-related Grade III-IV ASE in all of the treatment protocols: hypertension in a patient with a prior history. All therapy-related Grade I-II ASE listed with numbers of patients affected: hirsuitism and dyspepsia 4; headache, insomnia, nausea and tremor 2; hypertension, blurred vision, diarrhea, peripheral oedema, myalgia, parasthesiae, gum hypertrophy, cramps, chills and mood swings 1.
There were no therapy-related SAE although five patients required hospital admission: three during treatment phase (social 1, bleeding due to thrombocytopenia 2) and two over 12 months following therapy (atrial fibrillation with prior history and acute renal failure due to non-steroidal anti-inflammatory drugs). One male patient aged 85 was diagnosed with prostate cancer 18 months following treatment with TT4 and has been successfully treated. Other therapy-unrelated AE included renal colic, gum bleeding, easy bruising, back pain and menorrhagia.
Response rates
| . | TT4 (n=11) . | R1 (n=6) . | R2 (n=9) . | Overall triple therapy (n=26) . |
|---|---|---|---|---|
| Median time to response, days [range] | 7 [2-11] | 7 [4-14] | 23 [1-50] | 7 [2-50] |
| 6 month RR, % | 71 | 60 | 88 | 75 |
| 6 month CR, % | 29 | 40 | 63 | 45 |
| 12 month RR, % | 71 | 75 | 88 | 79 |
| 12 month CR, % | 43 | 50 | 50 | 47 |
| . | TT4 (n=11) . | R1 (n=6) . | R2 (n=9) . | Overall triple therapy (n=26) . |
|---|---|---|---|---|
| Median time to response, days [range] | 7 [2-11] | 7 [4-14] | 23 [1-50] | 7 [2-50] |
| 6 month RR, % | 71 | 60 | 88 | 75 |
| 6 month CR, % | 29 | 40 | 63 | 45 |
| 12 month RR, % | 71 | 75 | 88 | 79 |
| 12 month CR, % | 43 | 50 | 50 | 47 |
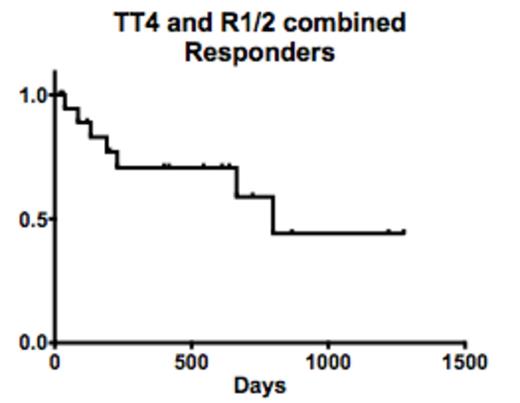
Relapse free survival. No effect by number of previous therapies or time between diagnosis and therapy.
Relapse free survival. No effect by number of previous therapies or time between diagnosis and therapy.
Successful depletion of peripheral blood B-cells from the low dose rituximab TT4 study. Mean at baseline 0.44x109/L [normal range 0.16-0.36], day 28 <0.01x109/L, 6 months 0.03x109/L and 12 months 0.08x109/L
Successful depletion of peripheral blood B-cells from the low dose rituximab TT4 study. Mean at baseline 0.44x109/L [normal range 0.16-0.36], day 28 <0.01x109/L, 6 months 0.03x109/L and 12 months 0.08x109/L
We report on the efficacy, safety and tolerability of a triple therapy approach to treating adult ITP. Notwithstanding the bias of non-random patient selection, we targeted B-cells, T-cells and antigen-presenting cells without the introduction of serious adverse events over the median follow-up period of 15.8 months across all arms. Overall, the 6 month response rate of 75% appears to be maintained beyond 12 months. Ongoing studies are required to confirm the safety of triple therapy approach to ITP. Although the median 7 days time to response seen in triple therapy compares favourably with other regimens, three patients required salvage therapy while awaiting their final doses of rituximab highlighting the need to further optimise sequential dosing strategies in future studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

![Figure 2. Successful depletion of peripheral blood B-cells from the low dose rituximab TT4 study. Mean at baseline 0.44x109/L [normal range 0.16-0.36], day 28 <0.01x109/L, 6 months 0.03x109/L and 12 months 0.08x109/L](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/21/10.1182_blood.v122.21.1084.1084/3/m_1084f02.png.jpeg?Expires=1767746233&Signature=hvMfSImoXVklb4u8Pyx3kOj1yz4kkVk8ygEvioppl~IWH9gWzy0Tab3Zf9GJjAIuN51OuW3euDQnBJO1etYaDm8okgLWcMYWCK-4Bie-zkP6hJZe3AULKyrdvx3uKLaWeFF1IR9TkWiIQfVgBGYaUR~k0FOOY4tpm~SF~y-AZwJDn1433f6QNS6sBP5MomzAgI9M0ywPtenvb8juPb4DM9euBaMTcG0kUNPtii7Iw8qoMyTPhnRvt1rveATmI3JAEIBl57-eTfuHpsjNRAx2fJbqSYXi3jfbKfDYLIh~IPqlCBeiE86sxQVlsHg4dFTs3xlTmu-JEYZe9zF1qWKdpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal