In this issue of Blood, Harper and colleagues explain how the mutation responsible for a common hereditary elliptocytosis results in the instability of the membrane and the shape of the cell. The conformational equilibrium of the spectrin dimers in these cells favors the “closed” state, with a consequent inhibition of tetramer formation, loss of membrane skeletal continuity, and shear resistance of the membrane.1
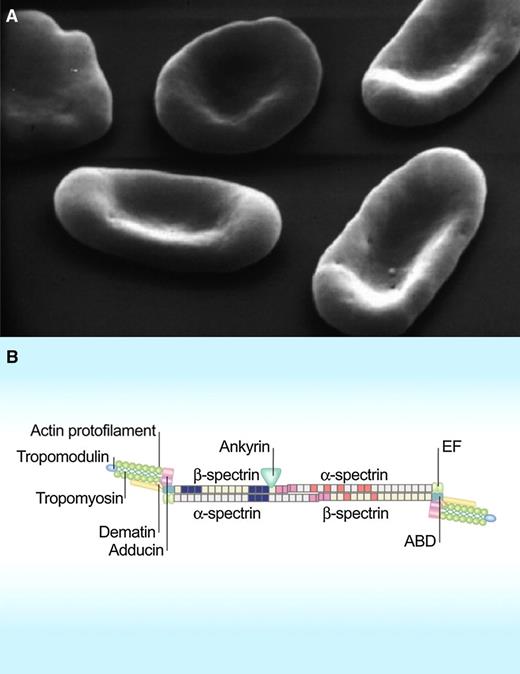
Scanning electron micrographs of red cells in hereditary elliptocytosis (A). Lateral linkages between spectrin dimer-dimers and between spectrin, actin, and protein 4.1R in the junctional complex in the spectrin-based red cell membrane skeleton (B). ABD, actin-binding domain; EF, hand motif. (Modified from Mohandas N, Gallagher PG. Blood. 2008;112(10):3939-3948.)
Scanning electron micrographs of red cells in hereditary elliptocytosis (A). Lateral linkages between spectrin dimer-dimers and between spectrin, actin, and protein 4.1R in the junctional complex in the spectrin-based red cell membrane skeleton (B). ABD, actin-binding domain; EF, hand motif. (Modified from Mohandas N, Gallagher PG. Blood. 2008;112(10):3939-3948.)
The unique shape and mutability of the red cell have perplexed and fascinated hematologists ever since Antonie van Leeuwenhoek observed in 1675 that: “when he was greatly disordered, the globules of his blood appeared hard and rigid, but grew softer and more pliable as his health returned: whence he infers that in a healthy body they should be soft and pliable.” Altered red cell shape is routinely used to classify red cell membrane disorders, such as hereditary spherocytosis and hereditary elliptocytosis. The past few decades have seen much progress in our understanding of the mechanistic and structural basis of these disorders.2 We now know that the elliptocytic phenotype is the result of weakening of the lateral interactions between skeletal proteins, most often between the pairs of spectrin dimers that make the tetramers (see figure).
What is less appreciated is the complex behavior of red cells during flow in vivo, when they experience a large range of fluid shear stresses in the vasculature, and in particular the contribution of the continuous “tank-tread” motion of the membrane during flow through the capillary bed.3-5 Although the structural perturbation of the red cell skeleton during tank treading is not fully defined, there is evidence of constant dissociation and reassociation of spectrin tetramers during induced membrane deformation.6 Thus a mutation that alters the propensity of spectrin dimers to associate to tetramers, or for spectrin tetramers to transiently dissociate in response to deformation, will influence the capacity of the membrane to undergo flow-induced dynamic deformations.
A universal feature of hereditary elliptocytosis associated with any of the known mutations in α- and β-spectrin is the reduced ability of the spectrin dimers with mutant subunits to form the tetramers that regulate deformability and mechanical stability of the red cell membrane.2,7 Although many of the identified mutations reside in domains of spectrin subunits directly involved in dimer-dimer contact interfaces account for the reduced proportion of tetramers in elliptocytes,8 a puzzling aspect is that some of the identified mutations, such as L260P in α-spectrin, lie far from the dimer contact site. It is in this context that the explanation by Harper et al of the manner in which an α-spectrin mutation, remote from the dimer self-association site, can cause a perturbation of that site is of such interest. This mutation (L260P) occurs in a common form of hereditary elliptocytosis. The results show that the mutation increases the stability of the closed conformation of the spectrin-dimer and thereby shifts the dimer-tetramer equilibrium in favor of the dimer, preventing the effective reformation of tetramers dissociated by shear.
In spite of the progress toward an understanding of hereditary elliptocytosis, several questions remain. Why and how do alterations in spectrin tetramer dynamics result in elliptocytic morphology? Are repeated passages of the cell in the microcirculatory bed necessary for the acquisition of the elliptocytic shape, and, if this is the case, does the ellipticity of the red cells increase with their time in circulation? To what extent does decreased proportion of tetramers contribute to altered tank treading behavior of the cell, and do these changes alter the effectiveness of oxygen delivery?
What are the implications of the findings of Harper and colleagues? One is that they provide new insight into how conformational changes induced by mutations far from the contact sites can alter spectrin tetramer–dimer balance, which reveals promoting the closed dimer conformation as a previously unsuspected mechanism for destabilization of the red cell membrane in hereditary elliptocytosis. In addition, the development of a mini-spectrin recombinant protein, affords a new means of studying of spectrin dimer–tetramer dynamics is a significant technical achievement, likely to prove valuable insights into furthering our understanding of red cell membrane properties. Importantly, the findings also reinforce the concept that red cell membrane protein interactions are dynamic and subject to perturbation by hydrodynamic forces that the cells encounter in the circulation. But in the broader context, the conclusions may also bear on the assembly and the structure-function relationships of other spectrin-like polymers.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal