Key Points
Blockmirs are designed against the miR-27 binding site in VE-cadherin and display restricted specificity.
Blockmirs regulate VE-cadherin and endothelial cell junctions, inhibit edema, and promote angiogenesis associated with ischemia.
Abstract
Cellular junctions are essential to the normal functioning of the endothelium and control angiogenesis, tissue leak, and inflammation. From a screen of micro RNAs (miRNAs) altered in in vitro angiogenesis, we selected a subset predicted to target junctional molecules. MiR-27a was rapidly downregulated upon stimulation of in vitro angiogenesis, and its level of expression is reduced in neovessels in vivo. The downregulation of miR-27a was essential for angiogenesis because ectopic expression of miR-27a blocked capillary tube formation and angiogenesis. MiR-27a targets the junctional, endothelial-specific cadherin, VE-cadherin. Consistent with this, vascular permeability to vascular endothelial growth factor in mice is reduced by administration of a general miR-27 inhibitor. To determine that VE-cadherin was the dominant target of miR-27a function, we used a novel technology with “Blockmirs,” inhibitors that bind to the miR-27 binding site in VE-cadherin. The Blockmir CD5-2 demonstrated specificity for VE-cadherin and inhibited vascular leak in vitro and in vivo. Furthermore, CD5-2 reduced edema, increased capillary density, and potently enhanced recovery from ischemic limb injury in mice. The Blockmir technology offers a refinement in the use of miRNAs, especially for therapy. Further, targeting of endothelial junctional molecules by miRNAs has clinical potential, especially in diseases associated with vascular leak.
Introduction
The tight control of vascular permeability is one of the chief functions of the endothelial lining of blood vessels. A loss of this barrier function of endothelium underlies many general and organ-specific disease processes, including leakiness of tumor vessels, various respiratory distress syndromes, complications of chemotherapy, and acute anaphylactoid reactions.
The controls of permeability generally fall into 2 classes: (1) the stimuli, such as thrombin, histamine, and vascular endothelial growth factor (VEGF), which induce leakiness1-3 ; and (2) a class of molecules such as angiopoetin-1 and sphingosine kinase,4,5 which exert a tonic effect on sustaining the status quo. Ultimately, these 2 influences converge on the cell-cell junctional molecules, such as VE-cadherin and PECAM, that regulate junctional structure and downstream signaling events.6
Given the relatively clear phenotypes induced by these stimuli, it is surprising how self-limited the leakiness is under the circumstances of repair or physiological angiogenesis. We reasoned that there may exist yet undiscovered mechanisms that will operate to force a quick restoration to the normality of vessels, for example in the process of angiogenesis or to operate during physiological angiogenesis to limit the leakiness of these newly forming vessels. This would be in stark contrast to tumor angiogenic vessels characterized by excessive leakiness, where these control mechanisms may be bypassed.
Because miRNA as a class of molecules have been especially adapted to mediate negative (and sometimes positive) feedback loops, our attention turned to this class of regulators. MiRNAs are small, single-stranded, noncoding RNAs that regulate both messenger RNA (mRNA) degradation and translation, at least partially through their ability to bind to the 3′UTR of target genes through base pairing with the 5′-end of the miRNA, through the so-called “seed sequence.”7 MiRNAs are predicted to alter the expression of a large set of proteins and have been linked to the control of complex physiological8 and pathophysiological processes. In the vasculature, they have been linked to regulation of development and to diseases such as tumor growth and cardiovascular disease.9-12 For example, the endothelial cell (EC)–restricted miRNA, miR-126, is highly expressed during vascular development and tumor angiogenesis, targeting, among other genes, the anti-angiogenic gene, SPRED-1,13-15 whereas miR-101 is downregulated in tumor vessels, acting through histone-methyltransferase (EZH2) to promote angiogenesis.16 In ECs, miR-296 is VEGF responsive, and blockade of this miRNA inhibits tumor-associated angiogenesis.17 MiR-132 is induced in tumor angiogenic vessels, targeting p120RasGAP, which acts downstream of integrins to increase cell proliferation and vascular growth.18 MiR-92a targets integrin signaling, and its inhibition improves blood flow recovery after ischemic insult.19
Although miRNAs have been proposed as a new class of therapeutic targets, they have one major feature that may limit their use: the large number of potential proteins that they regulate.20 This may be beneficial, for example if the targets are all involved in a specific signaling pathway, or they may be detrimental, for example if they have a high diversity in their effects. To circumvent this potential problem, the possibility arose for the use of inhibitors designed against the miRNA binding site in the 3′UTR of the specific target gene of interest. Such molecules would block the interaction of the miRNA with its specific binding site in the target protein and can therefore be viewed as Blockmirs.
Because cell-cell interactions are a critical component of EC function and are altered with the induction of angiogenesis, we investigated miRNAs regulated during capillary tube formation in a 3-dimensional type-1 collagen gel,21-23 which may target cell-cell junctional molecules. Here we describe the effects of one miRNA, miR-27a, that is downregulated during the angiogenic process and that has notable effects both on the junctional molecule, VE-cadherin, and on vascular permeability. We also show that a Blockmir, designed against the miR-27 binding site in the 3′UTR of VE-cadherin, is highly restricted to VE-cadherin and regulates and promotes VE-cadherin localization at cell-cell junctions. Further, this Blockmir has effects on VE-cadherin–dependent functions to inhibit EC permeability in vitro and vascular permeability in vivo. The Blockmir given as a single bolus injection promotes recovery from ischemia in an animal model of ischemic insult to the hindlimb, inhibiting vascular leak and promoting angiogenesis.
Materials and methods
Laser capture
Excision of ECs from the venules and neo-angiogenic vessels was achieved using the Arcturus PixCell IIe instrument. The laser diameter was set to 7.5 µM and the laser pulse was set at 0.2 s. Endothelial cells were transferred onto a CapSure Macro LCM Caps. Approximately 5 to 10 laser capture microdissection (LCM) caps were collected per patient for the 2 EC populations. Images were acquired at room temperature using UPlanFl 4×/0.13, UPlanFl 10×/0.30, and LCPlanFl 20×/0.40 objectives on a Arcturus PixCell IIe microscope (Molecular Devices) and acquired with a Hitachi ½-inch single-chip CCD color camera (Hitachi). Images were adjusted for brightness and contrast using LCM version 2.0 software.
Mouse model of unilateral hindlimb ischemia
At 7 weeks of age C57BL/6 mice underwent unilateral hindlimb ischemia as previously described,24 with minor modifications. With the mice under methoxyflurane inhalation anesthesia, the femoral vein and superficial and deep femoral arteries were ligated with 7-0 silk sutures. The left femoral vessels were then excised down to the saphenous artery. A sham procedure was performed on the contralateral limb. Blockmirs were injected systemically via the tail vein at a dose of 30 mg/kg of body weight. Hindlimb blood flow was measured using a high-definition laser Doppler imager (moorLDI2-IR, Moor Instruments, UK). Laser Doppler blood flow (LDBF) measurements were taken at different time points of both the left and right hindlimb. During laser scanning, the mice were anesthetized with methoxyflurane and placed on a heating pad (37°C) to minimize variations caused by body temperature. The hindlimbs were scanned a minimum of 3 times. The percentage of the ischemic (left) over sham (right) hindlimb blood flow was calculated and averaged.
Determination of the capillary density
The mice were killed by cervical dislocation. The medial thigh adductor muscles of ischemic and nonischemic limbs were harvested and processed as frozen sections of 8-μm thickness. The sections were fixed in ice-cold acetone for 10 minutes and stained with a cocktail of antibodies including rat anti-mouse Laminin (1:1000, Abcam), anti-CD31 conjugated to phycoerythrin (1:200, Abcam), and anti–smooth muscle actin conjugated to fluorescein isothiocyanate (1:500, Abcam). The sections were washed and stained with secondary anti-rat conjugated to Alexa Fluor dye 350 (1:2000, Invitrogen Molecular Probes). At room temperature, 10 different random microscopic fields at ×200 magnification (Olympus LUCPLFLN 20×/0.45 objective) were taken from each animal using an Olympus IX71 microscope and an Olympus DP71 camera. The acquisition software used were DP Controller (version 3.1.1.267) and DP Manager (3.1.1.208), both from Olympus. The images were analyzed using ImageJ version 1.46a software. Capillary density was expressed as the number of capillaries per the number of myocytes.
Other methods are available in the supplementary information.
Statistics
Unless otherwise stated, the Student t test was performed.
Ethics
Human ethics approval was obtained from the Central Sydney Area Health Service, Royal Prince Alfred Hospital Human Ethics Committee, and Animal Ethics approval was obtained from the Animal Ethics Committees of The University of Sydney, Central Sydney Area Health Service Animal Welfare Committee or University of NSW as required. This study was conducted in accordance with the Declaration of Helsinki.
Results
Identification of regulated miRNAs during in vitro angiogenesis
A miRNA microarray (GEO accession number: GSE50437) was used to identify miRNAs regulated during capillary tube formation. A list of the 18 significantly regulated miRNAs is given in supplemental Table 1. Fourteen of these 18 miRNAs have now been shown to be important in angiogenesis, confirming the validity of our model. Potential targets of the most highly regulated miRNAs and those involved in regulation of permeability were investigated using Web-based target prediction algorithms including TargetScan,25 PicTar,26 and miRanda.27 Of particular interest was miR-27a, predicted to target VE-cadherin, the endothelial-specific calcium-dependent cell adhesion molecule, responsible for cell-cell interactions and adhesion in solid tissues,28 and for VEGF-mediated signaling.29 The 3′UTR of VE-cadherin contains a single predicted 8-mer site for miR-27, with an exact match at positions 2 to 8 of the mature miRNA followed by an “A” (the seed region + position 8). The miRNA microarray expression profile of miR-27a and the 2 other members of this miRNA cluster, miR-23a and miR-24, were confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) (supplemental Figure 1).
MiR-27a alters VE-cadherin expression
VE-cadherin levels were measured in miR-27a-mimic transfected cells and showed a significant decrease (25 ± 4%) in protein expression (Figure 1A-B), a decrease of similar magnitude in cell surface expression as analyzed by flow cytometry (Figure 1C) and mRNA (by 31 ± 7%) (Figure 1D). Conversely, knockdown of miR-27 (supplemental Figure 2) using anti-miR made of locked nucleic acids (LNA) resulted in an upregulation (40 ± 4%) of VE-cadherin protein expression (Figure 1E-F) and cell surface expression (Figure 1G).
MiR-27a Targets VE-cadherin. (A) Expression of VE-cadherin in control or miR-27a-mimic transfected cells after 48 hours. β-actin was used as a loading control. (B) The normalized expression of the mean of 5 independent human umbilical vein entothelial cell (HUVEC) lines ± standard error of the mean (SEM). *P < .05 cf control. (C) The level of surface VE-cadherin as assessed by flow cytometry. The solid line represents the control mimic; the dashed line represents the miR-27a mimic. This is the result of one experiment, similar to three performed. (D) The level of VE-cadherin mRNA expression 24 hours posttransfection with miR-27a mimic. Results normalized to β-actin are the mean of quadruplicate quantitative reverse-transcription polymerase chain reaction (qRT-PCR) reactions ± SEM from 5 independent HUVEC lines. *P < .05 cf control. (E) VE-cadherin expression from HUVEC 48 hours post transfection with control or LNA-27–transfected cells. (F) The mean of 3 independent HUVEC lines ± SEM is shown. ***P < .001 cf control. (G) The level of surface VE-cadherin as assessed by flow cytometry. The solid line represents the control LNA; the broken dashed line represents LNA-27. This is the result of one experiment similar to two performed. (H) Luciferase constructs containing the 3′UTR of VE-cadherin containing the putative miR-27a binding site (Wt, black bars) or a mutated miR-27a binding site (Mut, white bars), together with control or miR-27a mimic. Results represent the mean of triplicate transfections ± SEM from 4 independent experiments. *P = .01, Wt + miR-27a vs Mut + miR-27a. **P = .00001, Wt + control vs Wt + miR-27a.
MiR-27a Targets VE-cadherin. (A) Expression of VE-cadherin in control or miR-27a-mimic transfected cells after 48 hours. β-actin was used as a loading control. (B) The normalized expression of the mean of 5 independent human umbilical vein entothelial cell (HUVEC) lines ± standard error of the mean (SEM). *P < .05 cf control. (C) The level of surface VE-cadherin as assessed by flow cytometry. The solid line represents the control mimic; the dashed line represents the miR-27a mimic. This is the result of one experiment, similar to three performed. (D) The level of VE-cadherin mRNA expression 24 hours posttransfection with miR-27a mimic. Results normalized to β-actin are the mean of quadruplicate quantitative reverse-transcription polymerase chain reaction (qRT-PCR) reactions ± SEM from 5 independent HUVEC lines. *P < .05 cf control. (E) VE-cadherin expression from HUVEC 48 hours post transfection with control or LNA-27–transfected cells. (F) The mean of 3 independent HUVEC lines ± SEM is shown. ***P < .001 cf control. (G) The level of surface VE-cadherin as assessed by flow cytometry. The solid line represents the control LNA; the broken dashed line represents LNA-27. This is the result of one experiment similar to two performed. (H) Luciferase constructs containing the 3′UTR of VE-cadherin containing the putative miR-27a binding site (Wt, black bars) or a mutated miR-27a binding site (Mut, white bars), together with control or miR-27a mimic. Results represent the mean of triplicate transfections ± SEM from 4 independent experiments. *P = .01, Wt + miR-27a vs Mut + miR-27a. **P = .00001, Wt + control vs Wt + miR-27a.
Ectopic expression of miR-27a suppressed luciferase activity (33 ± 3%) when a luciferase reporter plasmid containing the entire 3′UTR of VE-cadherin was co-transfected into HEK293T cells. Mutation of the miRNA site was able to reverse the repression of luciferase activity (Figure 1H), confirming the direct interaction between miR-27a and the miR-27a binding site in the 3′UTR of VE-cadherin.
MiR-27a alters VE-cadherin–dependent function
The effect of miR-27a overexpression (miR-27a-mimic) was tested in settings where VE-cadherin is known to be critically involved. MiR-27a-mimics had no effect on cell viability and induced a small but significant increase on EC proliferation (supplemental Figure 3). In EC monolayers, cells transfected with a control mimic showed that the characteristic closely apposed junctions decorated with VE-cadherin (Figure 2Ai). In contrast, miR-27a-mimic cells had gaps between the junctions (arrows) and there was a broader pattern and open zipper–like staining for VE-cadherin, indicative of a loose junction (Figure 2Aii). Cells transfected with anti-miR-27 also induced a change in VE-cadherin distribution. In a loosely packed monolayer, the control cells showed more intercellular gaps (arrows) and the open zipper–like staining for VE-cadherin (Figure 2Bi). In contrast, anti-miR-27 cells showed a smoother VE-cadherin staining, more tightly apposed junctions, and fewer intercellular gaps (Figure 2Bii). Consistent with the disrupted junctions, the miR-27a-mimic cells showed a small but significant increase in permeability (Figure 2C), whereas the anti-miR-27 transfected cells resulted in an inhibition of EC permeability after a transient stimulation with the powerful permeability-inducing agent, thrombin (Figure 2D). The anti-miR was also effective in vivo because it significantly reduced vascular permeability to VEGF in mice (Figure 2E) and, consistent with this result, the LNA also inhibited endogenous miR-27a levels in the tissue (Figure 2F). Finally, miR-27a-mimics inhibited capillary tube formation in vitro (Figure 3A) and angiogenesis in vivo using the Matrigel plug assay (Figure 3B).
MiR-27a alters VE-cadherin localization and EC permeability. (A-B) HUVEC stained for VE-cadherin 48 hours after transfection. (Ai) Control mimic or (Aii) miR-27a mimic; (Bi) control LNA or (Bii) LNA-27. The scale bar indicates 100 µm. Arrows indicate intercellular gaps. (C) Permeability measured in control or miR-27a-mimic–transfected cells after 48 hours. Results shown are the normalized means of 5 independent HUVEC lines ± SEM.*P < .05 cf control. (D) 48 hours after transfection with control LNA (black) or miR-27 LNA–transfected cells (white) permeability was measured without (NIL) or after thrombin stimulation (T). Results are from one experiment representative of three performed mean ± SEM *P< 0.05. (E) The Miles assay was performed with 4 µg of control (black) or anti-miR-27a (white bars) injected intradermally into the backs of the mice. 24 hours later, VEGF or phosphate-buffered saline (NIL) was given into the same site. *P < .05, **P < .005, n = 9 mice per group. (F) The levels of miR-27a compared with U6B as assessed in the skin tissue of mice given either control LNA + VEGF (black bar) or LNA-27 + VEGF (white bar). Results are the mean ± SEM of duplicate determinations from 3 mice per treatment. **P < .005
MiR-27a alters VE-cadherin localization and EC permeability. (A-B) HUVEC stained for VE-cadherin 48 hours after transfection. (Ai) Control mimic or (Aii) miR-27a mimic; (Bi) control LNA or (Bii) LNA-27. The scale bar indicates 100 µm. Arrows indicate intercellular gaps. (C) Permeability measured in control or miR-27a-mimic–transfected cells after 48 hours. Results shown are the normalized means of 5 independent HUVEC lines ± SEM.*P < .05 cf control. (D) 48 hours after transfection with control LNA (black) or miR-27 LNA–transfected cells (white) permeability was measured without (NIL) or after thrombin stimulation (T). Results are from one experiment representative of three performed mean ± SEM *P< 0.05. (E) The Miles assay was performed with 4 µg of control (black) or anti-miR-27a (white bars) injected intradermally into the backs of the mice. 24 hours later, VEGF or phosphate-buffered saline (NIL) was given into the same site. *P < .05, **P < .005, n = 9 mice per group. (F) The levels of miR-27a compared with U6B as assessed in the skin tissue of mice given either control LNA + VEGF (black bar) or LNA-27 + VEGF (white bar). Results are the mean ± SEM of duplicate determinations from 3 mice per treatment. **P < .005
Regulation of angiogenesis and vascular leak by miR-27. (A) HUVEC transfected with control (i and iii) or miR-27a mimic (ii and iv) and plated onto Matrigel. The retracted tubes are represented by a white arrow. The very thin tubes are represented by a black arrow. (i-ii) The scale bar indicates 200 µm. (iii-iv) The scale bar indicates 400 µm. (v) Number of capillary tubes formed per field of view, expressed as a percent relative to the control. *P < .05 cf control, n = 4 independent HUVEC lines, mean ± SEM. (B) Mice were implanted subcutaneously with Matrigel plugs containing fibroblast growth factor-2 and vehicle only (i,v), control (ii,vi), or miRNA-27a mimic (iii,vii). (i-iii) Representative histologic sections and hematoxylin and eosin–stained cross sections; the scale bar indicates 20 µm. (iv) Number of erythrocyte-containing vessels quantified. (v-vii) Representative CD31 immunochemistry. The scale bar indicates 50 µm. (viii) Number of CD31+ cells quantified. Data are expressed as mean ± SEM. Statistical analysis of differences was compared by 1-way analysis of variance with Bonferroni’s correction for multiple comparisons. Control (C) represents n = 3 mice and miRNA-27a and vehicle (V) represent n = 6 mice. (C) Reversal of the effect of miR-27a-mimics by overexpression of VE-cadherin. Cells were transfected with control mimic (i), miR-27a mimics (ii), VE-cadherin plasmid + control mimics (iii), or VE-cadherin expression plasmid + miR-27a-mimics (iv). 24 hours later, the cells were plated onto Matrigel and viewed over the following 24 hours. (D) Expression of miRNA-27a (open circles) and mRNA for VE cadherin (closed circles) over time after wounding monolayers of EC. Data are normalized to levels in the confluent cells. Expression levels were measured by qRT-PCR, with the results of miR-27a normalized to U48 and VE-cadherin normalized to β-actin. Results are from 2 to 4 independent HUVEC lines.
Regulation of angiogenesis and vascular leak by miR-27. (A) HUVEC transfected with control (i and iii) or miR-27a mimic (ii and iv) and plated onto Matrigel. The retracted tubes are represented by a white arrow. The very thin tubes are represented by a black arrow. (i-ii) The scale bar indicates 200 µm. (iii-iv) The scale bar indicates 400 µm. (v) Number of capillary tubes formed per field of view, expressed as a percent relative to the control. *P < .05 cf control, n = 4 independent HUVEC lines, mean ± SEM. (B) Mice were implanted subcutaneously with Matrigel plugs containing fibroblast growth factor-2 and vehicle only (i,v), control (ii,vi), or miRNA-27a mimic (iii,vii). (i-iii) Representative histologic sections and hematoxylin and eosin–stained cross sections; the scale bar indicates 20 µm. (iv) Number of erythrocyte-containing vessels quantified. (v-vii) Representative CD31 immunochemistry. The scale bar indicates 50 µm. (viii) Number of CD31+ cells quantified. Data are expressed as mean ± SEM. Statistical analysis of differences was compared by 1-way analysis of variance with Bonferroni’s correction for multiple comparisons. Control (C) represents n = 3 mice and miRNA-27a and vehicle (V) represent n = 6 mice. (C) Reversal of the effect of miR-27a-mimics by overexpression of VE-cadherin. Cells were transfected with control mimic (i), miR-27a mimics (ii), VE-cadherin plasmid + control mimics (iii), or VE-cadherin expression plasmid + miR-27a-mimics (iv). 24 hours later, the cells were plated onto Matrigel and viewed over the following 24 hours. (D) Expression of miRNA-27a (open circles) and mRNA for VE cadherin (closed circles) over time after wounding monolayers of EC. Data are normalized to levels in the confluent cells. Expression levels were measured by qRT-PCR, with the results of miR-27a normalized to U48 and VE-cadherin normalized to β-actin. Results are from 2 to 4 independent HUVEC lines.
To confirm that VE-cadherin is a major regulator of capillary tube formation, VE-cadherin was knocked down using siRNA, yielding approximately 20% to 30% knockdown, similar to that seen with the miR-27a mimics, whereas little effect was seen on ZO-2 expression (supplemental Figure 4A). The cells remained viable, but when they were plated onto Matrigel, the capillary tubes were thin and prone to breakage (supplemental Figure 4Bii) and no longer formed a stable network, as occurred in the control (supplemental Figure 4Bi). We then performed a rescue experiment on the miR-27-mimic cells using a VE-cadherin expression plasmid. An increase in approximately 30% in the amount of VE-cadherin expression was achieved with this expression plasmid (supplemental Figure 4C). VE-cadherin overexpression was able to partially reverse the effects of miR-27a-mimics in the capacity to form capillary tubes on Matrigel (Figure 3C). Finally, when we induced migration of EC using a scratch assay, miR-27a was rapidly downregulated, as expected, based on our array data (supplemental Table 1 and Figure 1), whereas the mRNA levels of VE-cadherin were upregulated. By 24 hours after wounding, miR-27a levels were high and consistent with this, the levels of VE-cadherin mRNA had decreased (Figure 3D), as had the protein (supplemental Figure 4D), as would be expected on sparse migrating cells. Together, these results show an inverse relationship between miR-27a and VE-cadherin mRNA, suggesting that VE-cadherin is a major target of miR-27a in ECs.
MiR-27a is downregulated in neovessels in disease
Our results suggest that the ECs in vessels undergoing a limited angiogenic response should have decreased levels of miR-27a compared with ECs in mature nonangiogenic vessels. To test this hypothesis, we investigated the expression of miR-27a in vessels from patients with a disease in which angiogenesis is known to occur but is not associated with tumor growth.30,31 Paraffin-embedded liver sections were obtained from 3 patients with cirrhosis. The fibrotic area surrounding the regenerative nodules is known to be a setting where new vessels form via angiogenesis (neovessels), and this area in normal liver is free from such neovessels.30 ECs from venules and neovessels (Figure 4A) were collected by LCM, and RNA was isolated from the samples. MiRNA profiles, analyzed using Taqman low-density arrays showed that similar numbers of miRNAs were detected in the venules as in the neovessels in each of the 3 patients, although there was variation in the number of miRNAs detected among patients. The average Ct value for detected miRNAs was similar between both groups and patients (supplemental Figure 5). In our original array screen, miR-520d* was found to be highly stable and therefore was used as a normalization control for the qRT-PCR. We were unable to investigate levels of mRNAs specific for ECs or hepatocytes because of the limitation of the amount of captured material. However, it was noted that miR-122, a highly abundant and liver-specific miRNA that accounts for 70% of the total liver miRNA population and that is undetectable in other tissues,31 was not detected in any of the samples, suggesting that the captured cells were not significantly contaminated by hepatocytes. The RNA samples were then analyzed by qRT-PCR for miR-27a expression and results were normalized to miR-520d* expression (Figure 4B). For all 3 patients, there was a significant decrease in miR-27a levels in cells from the neovessel population compared with cells from the venules. Thus, although the patient numbers are small, the data support the idea that downregulation of miR-27a is associated with regulated angiogenesis.
Regulation of miR-27a in human disease. Human liver tissue from 3 patients with cirrhosis was stained for CD31. (A) Neo-angiogenic vessels (neovessels) in the fibrous septa; (ii) the black circle and venules as indicated with black arrows were captured by LCM. The scale bars indicate (i) 15 µm and (ii) 30 µm. (B) RNA was isolated from ECs in either venules or neovessels (Neo) from three patients (1-3). Expression levels of miR-27a were quantified by qRT-PCR and normalized to miR-520d*. Data represent the mean of quadruplicate qRT-PCR reactions ± SEM. *P < .05 Venules vs Neo.
Regulation of miR-27a in human disease. Human liver tissue from 3 patients with cirrhosis was stained for CD31. (A) Neo-angiogenic vessels (neovessels) in the fibrous septa; (ii) the black circle and venules as indicated with black arrows were captured by LCM. The scale bars indicate (i) 15 µm and (ii) 30 µm. (B) RNA was isolated from ECs in either venules or neovessels (Neo) from three patients (1-3). Expression levels of miR-27a were quantified by qRT-PCR and normalized to miR-520d*. Data represent the mean of quadruplicate qRT-PCR reactions ± SEM. *P < .05 Venules vs Neo.
Blockmirs to VE-cadherin regulate VE-cadherin expression
MiRNAs are able to target multiple genes, at least partially because of the highly conserved seed sequence used in target binding.20 Thus, demonstration of a specific target being the critical protein involved in the function is difficult. The possibility of using a Blockmir technology, an inhibitor to the miRNA-binding site in a specific target gene, was therefore investigated. The structure of the Blockmirs and the antagomir and control is given in supplemental Figure 6 and is based on LNA and 2′O-methyl-RNA monomers. Blockmir CD5-2 consistently and significantly increased the level of VE-cadherin, similar to that seen with the anti-miR-27 (supplemental Figure 7). Blockmir CD5-2 regulated VE-cadherin localization to junctions in EC monolayers, converting the staining pattern from the loose open zipper staining with intercellular gaps (Figure 5Ai, arrows) to tighter staining patterns (Figure 5Aii), inhibited permeability to thrombin (Figure 5B), and inhibited VEGF-induced vascular leak in mice (Figure 5C).
Blockmirs regulate VE-cadherin–dependent functions. (A) HUVECs were stained for VE-cadherin 48 hours after transfection. (i) Control or (ii) CD5-2. White arrows indicate intercellular gaps. (B) Cells were transfected with control or CD5-2, and permeability was assessed through unstimulated or thrombin-stimulated monolayers. Results are normalized to permeability of control (n = 1). The mean ± SEM of 3 to 5 experiments is shown. **P < .005. (C) The Miles assay was performed in mice with control (black bars) or CD5-2 (white bars) injected intravenously. 24 hours later, VEGF or phosphate-buffered saline was given intradermally (n = 4 mice per group). **P < .005.
Blockmirs regulate VE-cadherin–dependent functions. (A) HUVECs were stained for VE-cadherin 48 hours after transfection. (i) Control or (ii) CD5-2. White arrows indicate intercellular gaps. (B) Cells were transfected with control or CD5-2, and permeability was assessed through unstimulated or thrombin-stimulated monolayers. Results are normalized to permeability of control (n = 1). The mean ± SEM of 3 to 5 experiments is shown. **P < .005. (C) The Miles assay was performed in mice with control (black bars) or CD5-2 (white bars) injected intravenously. 24 hours later, VEGF or phosphate-buffered saline was given intradermally (n = 4 mice per group). **P < .005.
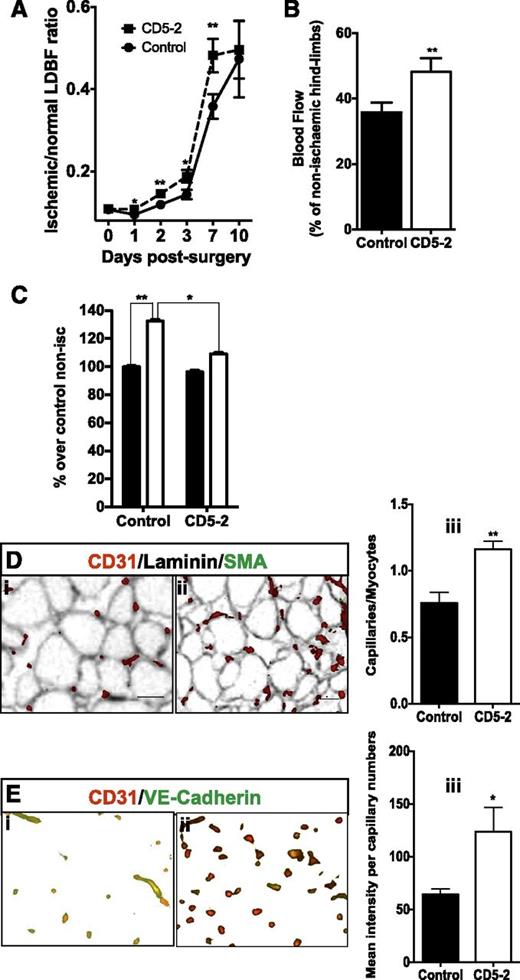
Finally, we investigated the effect of the CD5-2 on recovery after hindlimb ischemia. MiR-27a was rapidly downregulated within the first 2 days after injury and returned to normal or slightly higher levels by day 10 (supplemental Figure 8). Blockmir CD5-2 was given as a single systemic injection on day 0, immediately after the induction of ischemia, and led to a significant improvement in the blood flow over the course of the next 10 days (Figure 6A), and was particularly evident on day7 (Figure 6B). Moreover, the recovery from ischemia became evident within 24 hours (Figure 6A; supplemental Figure 9). CD5-2 resulted in a decrease in the edema in the ischemic muscle within the first 24 hours (Figure 6C) and stimulated angiogenesis, as assessed by CD31 staining after 7 days (Figure 6D). Finally, consistent with the targeting of the Blockmir to miR-27-VE-cadherin, there was an increase in the expression of VE-cadherin on the CD31-positive vessels within the ischemic muscle (Figure 6E). Of interest is the fact that in the nonischemic limb, CD5-2, although it did not promote an increase in the number of capillaries (supplemental Figure 10C), it did appear to increase the level of VE-cadherin in the capillaries (supplemental Figure 10D), although this did not reach statistical significance in the 5 mice analyzed. Because ischemic recovery is influenced by both the limitation of edema and the extent of angiogenesis,32 our results suggest that the Blockmir influences both of these aspects. The degree of recovery with CD5-2 is similar to that seen with other anti-miRNAs in this ischemic model, for example with anti-miR-100, which targets mTOR to promote angiogenesis.33
Blockmirs regulate edema and angiogenesis after ischemia. (A) Hindlimb blood flow expressed as a percentage of ischemic limb blood flow over nonischemic hindlimb blood flow measured 10 days after surgery. **P < .01 (n = 6-10 mice per group). The LDBF between days 0 and 7, representing the mean of 3 independent experiments. (B) The ischemic LDBF of control and CD5-2 at day 7 is represented as a percentage over non-ischemic hind-limb blood flow. CD5-2 is represented by dashed lines; controls are represented by solid lines. (C) Assessment of edema 24 hours after hindlimb ischemia for mice treated with control Blockmir (black bars, nonischemic) and CD5-2 (white bars, ischemic). The graph is expressed as a percentage of dye leakage in either the nonischemic or ischemic side of control and CD5-2–treated mice over dye leakage in nonischemic controls. The lower part of adductor muscle was taken for quantification *P < .05, **P < .01 (n = 8 per group, results pooled from 2 independent experiments). (D) Assessment of capillary density. Sections were stained for CD31. A representative area is shown for one mouse given (i) control and (ii) CD5-2. (iii) Quantification of the number of capillaries is given as the ratio of capillaries/myocytes in the ischemic limbs (n = 4-5 animals), *P < .02. (E) Sections were co-stained with CD31 (red) and VE-cadherin (green). A representative area of the ischemic region is shown for one mouse given (i) control and (ii) CD5-2. (iii) Mean pixel intensity of VE-cadherin is expressed relative to the number of capillaries, which are CD31+ (n = 4 animals), *P < .05.
Blockmirs regulate edema and angiogenesis after ischemia. (A) Hindlimb blood flow expressed as a percentage of ischemic limb blood flow over nonischemic hindlimb blood flow measured 10 days after surgery. **P < .01 (n = 6-10 mice per group). The LDBF between days 0 and 7, representing the mean of 3 independent experiments. (B) The ischemic LDBF of control and CD5-2 at day 7 is represented as a percentage over non-ischemic hind-limb blood flow. CD5-2 is represented by dashed lines; controls are represented by solid lines. (C) Assessment of edema 24 hours after hindlimb ischemia for mice treated with control Blockmir (black bars, nonischemic) and CD5-2 (white bars, ischemic). The graph is expressed as a percentage of dye leakage in either the nonischemic or ischemic side of control and CD5-2–treated mice over dye leakage in nonischemic controls. The lower part of adductor muscle was taken for quantification *P < .05, **P < .01 (n = 8 per group, results pooled from 2 independent experiments). (D) Assessment of capillary density. Sections were stained for CD31. A representative area is shown for one mouse given (i) control and (ii) CD5-2. (iii) Quantification of the number of capillaries is given as the ratio of capillaries/myocytes in the ischemic limbs (n = 4-5 animals), *P < .02. (E) Sections were co-stained with CD31 (red) and VE-cadherin (green). A representative area of the ischemic region is shown for one mouse given (i) control and (ii) CD5-2. (iii) Mean pixel intensity of VE-cadherin is expressed relative to the number of capillaries, which are CD31+ (n = 4 animals), *P < .05.
We investigated the specificity of the VE-cadherin–directed Blockmir against 2 other miR-27–verified targets, SEMA6A and PPARγ.34,35 Although antimiR-27 (LNA-27) induced an increase in VE-cadherin, SEMA6A, and PPARγ protein expression, consistent with these being target mRNAs, CD5-2 induced an increase only in VE-cadherin (Figure 7A-B; supplemental Figure 11A-B). We also used luciferase reporter assays to investigate the specificity. MiR-27a overexpression caused the expected inhibition of VE-cadherin luciferase activity (Figure 7C). CD5-2 reversed the miR-27a–mediated inhibition, whereas a control Blockmir did not. The LNA-27 also reversed the inhibition caused by miR-27a overexpression (supplemental Figure 12A). Mutation of the miR-27 site in VE-cadherin abolished the inhibition by CD5-2 (supplemental Figure 12B). Finally, miR-27a overexpression inhibited the luciferase activity of the construct containing the 3′UTR of PPARγ (Figure 7D), but CD5-2 did not reverse this inhibition. Together these experiments demonstrate a degree of selectivity in the design of the Blockmirs, with CD5-2 showing activity against VE-cadherin and not against SEMA6A and PPARγ. Further, it suggests that although the Blockmir binds to the miR-27 binding site in the 3′UTR of VE-cadherin, it does not activate the miRNA degradative machinery, presumably through lack of recruitment of the necessary accessory protein.
Specificity of the Blockmir. (A) Western blot analyses of VE-cadherin, SEMA6A from HUVECs transfected with the LNA27, CD5-2, or control; α-tubulin was used as a loading control. (B) Quantitative analysis as described in (A): control (white bar), LNA27 (hatched bar), and CD5-2 (black bar); mean ± SEM, *P < .05 (n = 3-5). (C) VE-cadherin and (D) PPARγ reporter activity from cells co-transfected with mimic control (Vector[−]) or miR-27a-mimic only (Vector), or miR-27a-mimic plus Blockmirs control or CD5-2. (C) Mean ± SD from 3 independent experiments with 3 replicate samples per experiment; analysis of variance *P ≤ .05. (D) *P < .05 from a representative experiment.
Specificity of the Blockmir. (A) Western blot analyses of VE-cadherin, SEMA6A from HUVECs transfected with the LNA27, CD5-2, or control; α-tubulin was used as a loading control. (B) Quantitative analysis as described in (A): control (white bar), LNA27 (hatched bar), and CD5-2 (black bar); mean ± SEM, *P < .05 (n = 3-5). (C) VE-cadherin and (D) PPARγ reporter activity from cells co-transfected with mimic control (Vector[−]) or miR-27a-mimic only (Vector), or miR-27a-mimic plus Blockmirs control or CD5-2. (C) Mean ± SD from 3 independent experiments with 3 replicate samples per experiment; analysis of variance *P ≤ .05. (D) *P < .05 from a representative experiment.
Discussion
The work described here enhances our understanding of miRNA control of EC biology. First, we show a potent effect of a miRNA on vascular leakiness. The inhibition of miR-27a inhibits vascular leak to a variety of stimuli, including thrombin (Figure 2D), VEGF (Figure 2E), and, most importantly, as part of injury caused by arterial vascular obstruction (Figure 6). To our knowledge, this is the first example of an miRNA inhibiting a wide spectrum of vascular leaks and, given the paucity of agents that control this pathogenic phenomenon, it has interesting clinical implications.
The second novel aspect is the targeting of VE-cadherin by miR-27a. MiRNAs have, by their very nature, a multiplicity of targets, and it would be inappropriate to conclude that all of the effects are caused by changes in VE-cadherin. However, this is a central molecule in the regulation of cell junctions and vascular function. VE-cadherin knockout mice show embryonic lethality and, although ECs are still able to form the primary vascular plexus, vascular remodeling is deficient.36 VE-cadherin is also a critical regulator of mature EC function.6,28,37-39 The angiogenic response under physiological conditions is associated with increases in VE-cadherin phosphorylation and changes in localization, which are associated with migration and increases in vascular permeability.39,40 Here we show that miR-27a levels are inhibited early in the angiogenic response, and this downregulation is necessary for angiogenesis. We show an inverse relationship between miR-27a levels and VE-cadherin mRNA. This, together with the effects of miR-27a overexpression or inhibition, is consistent with miR-27a targeting VE-cadherin, and VE-cadherin reconstitution experiments reversed the angiogenic phenotype induced by the miR-27a-mimics. Although the regulation of VE-cadherin protein levels by miR-27 is small (∼20-30%), evidence is accumulating that in general miRNAs “fine-tune” their targets by <50%.41 This degree of downregulation is consistent with the function of VE-cadherin, where partial changes in VE-cadherin expression significantly affect vascular function without influencing cell survival.42 This suggests that the levels of VE-cadherin must be finely controlled.
The third important aspect we show here is the use of a novel technology that enhances the specificity of anti-miRs. Blockmirs are designed to bind to the seed together with additional bases at either end of the target to generate the specificity. Blockmir CD5-2 targeted VE-cadherin, stabilized cell-cell junctions, and regulated vascular leakage. Further, this Blockmir enhanced recovery after ischemia, even when it was delivered as a single bolus intravenous injection at the time of the ischemic insult. Recovery was associated with an inhibition of edema and an enhancement of the angiogenic response. Our results with the Blockmir represent a major step, because miRNAs are becoming subject to an increasing number of clinical trials and offer an alternative design that may have significant advantages owing to increased specificity.
Finally, our results shed light on the complexity of control processes by miRNAs. Inhibition of miR-27 has been described previously to inhibit angiogenesis in a choroidal neovascular model, and this correlated with an increase in expression of 2 anti-angiogenic molecules, Sprouty2 and Sema6A.34 Inhibition of miR-27 can also inhibit angiogenesis through inhibition of sprout formation, achieved through Sema6A regulation43,44 and Δ-like ligand 4.44 Our results here show that angiogenesis can also be inhibited by overexpression of miR-27a with the in vitro reconstitution experiments, confirming the target as VE-cadherin. We also demonstrated that inhibition of miR-27 activity promotes angiogenesis and, in a setting of a “controlled” angiogenic response as is seen in liver fibrosis, miR-27a levels are decreased. Thus our results together with those previously reported suggest a number of pertinent points. First, they demonstrate that there are many key angiogenic proteins that are targeted by miR-27 to regulate angiogenesis. This further suggests that miR-27a modulates the expression of different genes important through the different phases of the angiogenic process, rather than operating as purely an “on-off” signal. This is similar to that suggested for miR-126.13,14 Second, under normal angiogenesis, the regulation of the levels of miR-27a must be exquisitely controlled because both prolonged increases or decreases (as is achieved by mimic or LNA delivery) perturb angiogenesis. The factors that induce changes in miR-27a levels are not well understood. Our preliminary studies suggest that downregulation is seen in a 3-dimensional setting as was used in our initial collagen gel tube-forming assay and that VEGF and fibroblast growth factor under 2-dimensional conditions have no effect. In addition, Urbich et al12 also show no changes in miR-27 with VEGF but see an upregulation under laminar flow. Finally, in pathologies, such as tumors, where high levels of miR-27 are seen, miR-27a may function to enhance angiogenesis through decreasing the expression of the inhibitors Sprouty2 and Sema6A and destabilizing the vessels through inhibition of VE-cadherin. Consistent with this, tumor vessels have uncontrolled growth and are known to be highly leaky.13 Thus, should miR-27 be considered as a therapeutic, the dilemma over its multiple target proteins needs to be considered. We have begun to address this issue with the use of the Blockmirs. The specificity of the activity of the Blockmir CD5-2 was demonstrated by its selective regulation of VE-cadherin, either of protein expression or transcriptional activity in luciferase reporter assays, and not 2 other verified targets, PPARγ and SEMA6A. Although miR-27 is predicted to target many hundreds of genes, the demonstration that LNA-27 targets all 3 genes, but the Blockmir CD5-2 selectively targets VE-cadherin, demonstrates a high degree of selectivity in the design of this Blockmir.
In summary, not only is miR-27a a major regulator of angiogenesis, it also regulates EC junctions to control vascular integrity. Thus inhibition of miR-27 or its specific interaction with VE-cadherin, as demonstrated with the use of the Blockmir, inhibits vascular leak in the absence of angiogenesis as demonstrated in the Miles assay. To our knowledge, this is the first miRNA described that functionally and biochemically regulates cell-cell interactions and can serve as an antipermeability agent. Because vascular leak is a chief pathophysiological mechanism of many vascular, inflammatory, and neoplastic diseases, miR-27a maybe a novel target in the development of antipermeability therapies. The demonstration of a specific antagomir that can block the miR-27–VE-cadherin interaction, without activating the miRNA machinery, should aid in this therapeutic development.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE50437).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (571408), the National Heart Foundation, and the Wenkart Foundation (J.A.Y.). J.R.G. holds the Wenkart Chair of the Endothelium, Sydney Medical School, The University of Sydney.
Authorship
Contribution: J.A.Y., K.K.T., and J.L. performed the experiments and contributed to some aspects of the manuscript writing; J.R.G. and M.A.V. devised the project and wrote the manuscript; and T.M., L.D., Y.L., A.J.L., J.M., L.P.-L., L.M.K., M.N., P.A.G., G.J.G., A.T., I.L., C.N.H., N.T., N.S., J.G.K., and G.M. contributed expertise for specific aspects of the investigation.
Conflict-of-interest disclosure: T.M. is a consultant and holds stock in Mirrx Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer R. Gamble or Mathew A. Vadas, Centenary Institute, Locked Bag #6, Newtown 2042, NSW, Australia; e-mail: j.gamble@centenary.org.au or m.vadas@centenary.org.au.
References
Author notes
J.A.Y., K.K.T., and J.L. contributed equally to this study.






![Figure 7. Specificity of the Blockmir. (A) Western blot analyses of VE-cadherin, SEMA6A from HUVECs transfected with the LNA27, CD5-2, or control; α-tubulin was used as a loading control. (B) Quantitative analysis as described in (A): control (white bar), LNA27 (hatched bar), and CD5-2 (black bar); mean ± SEM, *P < .05 (n = 3-5). (C) VE-cadherin and (D) PPARγ reporter activity from cells co-transfected with mimic control (Vector[−]) or miR-27a-mimic only (Vector), or miR-27a-mimic plus Blockmirs control or CD5-2. (C) Mean ± SD from 3 independent experiments with 3 replicate samples per experiment; analysis of variance *P ≤ .05. (D) *P < .05 from a representative experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/16/10.1182_blood-2012-12-473017/4/m_2911f7.jpeg?Expires=1765924522&Signature=w70r22257aM6SdizpCB5JYAhRuS3~JX3T5OInjwS2VvP9HHMS87Q-nVrvbRtdUpV5IMtcuTVRJJX30pL45Q4N-xNO-A7g~Qe3DJ21lWK-AOrTjAqBwY8Z5RkCiazOPA70Fi~vJnyscKKLYTnNPxlDPsFkK1QkjVAWyqdo5aW56i8gjfCTmaBWjzHejxX3B-Ft-ANXuxPfTrumqtnNDGEI9sBLB1oSBUsdxwwlyyk2bZ~6z01b2P~0rydqAubTGtbxw61bu7C46NkszclEX5UIMdZR4NOhI0qEW2k11fGbU-r6lw5w3IPDhsMs~oXf-3cYaC9yJcn~pw62rwnN7Pfrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal