Key Points
IκB-ζ is essential for nuclear NF-κB activity in ABC DLBCL.
ABC DLBCL survival depends on IκB-ζ signaling.
Abstract
Constitutive activation of the nuclear factor-κ B (NF-κB) pathway is a hallmark of the activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL). Recurrent mutations of NF-κB regulators that cause constitutive activity of this oncogenic pathway have been identified. However, it remains unclear how specific target genes are regulated. We identified the atypical nuclear IκB protein IκB-ζ to be upregulated in ABC compared with germinal center B-cell–like (GCB) DLBCL primary patient samples. Knockdown of IκB-ζ by RNA interference was toxic to ABC but not to GCB DLBCL cell lines. Gene expression profiling after IκB-ζ knockdown demonstrated a significant downregulation of a large number of known NF-κB target genes, indicating an essential role of IκB-ζ in regulating a specific set of NF-κB target genes. To further investigate how IκB-ζ mediates NF-κB activity, we performed immunoprecipitations and detected a physical interaction of IκB-ζ with both p50 and p52 NF-κB subunits, indicating that IκB-ζ interacts with components of both the canonical and the noncanonical NF-κB pathway in ABC DLBCL. Collectively, our data demonstrate that IκB-ζ is essential for nuclear NF-κB activity in ABC DLBCL, and thus might represent a promising molecular target for future therapies.
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most frequent malignant lymphoma subtype, accounting for approximately 30% to 40% of all cases. DLBCL is a heterogeneous diagnostic category with respect to morphology, clinical manifestation, and prognosis.1 Gene expression profiling revealed the existence of 2 major molecular DLBCL subtypes, termed germinal center B-cell-like (GCB) and activated B-cell–like (ABC) DLBCL, which are discriminated by differences in gene expression and clinical outcome.2
ABC DLBCLs are characterized by constitutive activation of the nuclear factor-κ B (NF-κB) pathway.3,4 The NF-κB transcription factor family consists of 5 structurally related members: RelA (p65), RelB, c-Rel, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100).5-8 In resting cells, these proteins are kept inactive in the cytoplasm by binding to the inhibitory IκB proteins. On stimulation, the NF-κB members are released from the IκBs and translocate into the nucleus, where they activate their target genes.5,6 Activation of NF-κB occurs either through the canonical or the noncanonical (alternative) pathways. Among others, stimulation of the B-cell receptor, Toll-like receptor, or tumor necrosis factor (TNF) receptor activates the canonical NF-κB pathway, whereas stimulation of the B-cell activating factor receptor or CD40 engages the noncanonical pathway.9
In ABC DLBCL, various somatically acquired mutations have been identified that lead to constitutive activation of the NF-κB pathway. These mutations affect positive (CARD11, CD79A, CD79B, and MYD88) and negative (TNFAIP3 [A20]) NF-κB regulators.4,10-13 Although the molecular mechanisms leading to constitutive NF-κB signaling are relatively well characterized, little is known about the contribution of selective NF-κB and IκB family members to the control of the target gene repertoire.
IκB-ζ (also known as MAIL), encoded by NFKBIZ, is an atypical nuclear member of the IκB family.14-16 In contrast to classical IκB proteins that are constitutively expressed and controlled by inducible degradation, IκB-ζ expression is barely detectable in resting cells but is rapidly induced by different pro-inflammatory stimuli such as lipopolysaccharides.17,18 IκB-ζ has been shown to regulate NF-κB signaling, as reporter analyses suggested that IκB-ζ may act as an inhibitor of NF-κB.14 In contrast, other studies have reported that IκB-ζ can induce gene expression of individual NF-κB target genes such as IL-6.19
A recent array comparative genomic hybridization (aCGH) study identified recurrent genomic amplifications of the NFKBIZ locus that occurred in roughly 10% of ABC DLBCL cases, suggesting a role of IκB-ζ in the pathogenesis of this subtype.20 We therefore elucidated the functional role of IκB-ζ in ABC DLBCL biology.
Material and methods
Cell culture, retroviral constructs, and transductions
Human DLBCL, Burkitt lymphoma (BL), multiple myeloma (MM), and classical Hodgkin lymphoma (cHL) cell lines were cultured in RPMI (Invitrogen) with 10% fetal calf serum (Sigma-Aldrich) except for OCI-Ly1, OCI-Ly2, OCI-Ly3, OCI-Ly7, OCI-Ly10, and TMD8, which were cultured in Iscove’s modified Dulbecco medium supplemented with either 20% human plasma or 10% fetal calf serum. All cell lines were maintained at 37°C with 5% CO2.
For retroviral transductions, all cell lines were engineered to express the murine ecotropic retroviral receptor, as described.21 To allow doxycycline-inducible small hairpin RNA (shRNA) or cDNA expression, all cell lines except for L363 were modified to express the bacterial tetracycline repressor, as described.21 To assess toxicity of an shRNA, retroviruses that coexpress green fluorescent protein (GFP) were used as described.21 In brief, flow cytometry was performed 2 days after shRNA transduction to determine the initial GFP-positive proportion of live cells for each shRNA. Subsequently, cells were cultured with doxycycline to induce shRNA expression and sampled over time. The GFP-positive proportion at each time was normalized to that of the negative control shRNA and further normalized to the day 2 fraction. Each shRNA experiment was reproduced independently at least 3 times for each cell line. As a positive shRNA control, we used previously described shRNAs against CARD11 for ABC DLBCL and MYC for GCB DLBCL cell lines.21 The target sequence of IκB-ζ shRNAs 1 and 2 were GCAGTCCTGATGTATCTGTAC and GTGTCCATGGTTAGAATTTGA, respectively. As a negative control, we used a previously described shRNA directed against methylsterol monooxygenase 1.21 For the shRNA rescue experiment, HBL-1 cells that expressed an IκB-ζ cDNA (NM_031419.3) were created, and the experiment was performed as described.21 The rescue experiment was reproduced 3 times.
To knockdown MYD88 expression, a previously described MYD88 shRNA was used.12 Expression of CARD11 (NM_032415.3) and MYD88 (NM_002468.4) wild-type and previously described mutants was achieved by cloning the corresponding cDNAs into a modified version of the pRetroSUPER dual-promoter vector, as described.10,12
aCGH
aCGH in DLBCL cell lines was performed as described.21 aCGH data were obtained from the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/geo) through GEO accession number GSE43272.
Gene expression profiling
Gene expression data from 350 DLBCL patient samples that were profiled using Affymetrix HG-U133 Plus 2.0 microarrays by Lenz et al22 were obtained from GEO (accession number GSE10846). Patients were classified into ABC, GCB, and unclassified DLBCL according to Lenz et al.22 Gene expression data from MM, cHL, and BL, as well as ABC and GCB DLBCL patient samples, were obtained from GEO (GSE2658, GSE12453, GSE14879, GSE40160, and GSE4732).23-25 To take considerable differences in brightness distributions of these cohorts into account, we quantile-normalized all microarray experiments on the basis of all identical Affymetrix probe sets to minimize the offsets between the cohorts. Two-sample t tests were used to compare IκB-ζ expression in each lymphoma subtype to IκB-ζ expression detected in ABC DLBCL.
Gene expression profiling was performed 6, 12, and 24 hours after treatment of HBL-1 cells with the IKKβ inhibitor MLN120b, as well as 24, 48, 72, and 96 hours after induction of IκB-ζ shRNA 1 in HBL-1 cells. Total RNA was isolated using the NucleoSpin RNA II kit (Macherey & Nagel), according to the manufacturer's protocol. RNA was amplified and labeled with the TotalPrep RNA Amplification Kit (Illumina). Samples were subsequently hybridized on HumanHT-12 v4 Expression BeadChips (Illumina), following the manufacturer's protocol. Changes in gene expression induced by MLN120b treatment and IκB-ζ knockdown were measured in 2 independent biological replicates. Gene expression profiling after treatment with the mucosa-associated lymphoid tissue lymphoma translocation gene 1 inhibitor z-VRPR-fmk was performed using whole-genome Agilent 4 × 44K gene expression arrays (Agilent Technologies). The analysis algorithm is described in supplemental Materials and methods (available on the Blood Web site). The gene expression data have been deposited in the GEO database under accession number GSE46974.
Polymerase chain reaction (PCR) amplification and sequencing
PCR amplification of NFKBIZ exons and sequencing was performed as previously described.10
Quantitative PCR
To determine IκB-ζ, IL-6, IL-10, SOCS3, and NFKBIA mRNA expression levels, quantitative PCR was performed as previously described,20 using predesigned assays (Applied Biosystems).
Western blotting
Western blotting was performed as described.21 Fractionated nuclear and cytoplasmic proteins were prepared using the Nuclear Extract Kit (Active Motif). The protein amount was quantified using the BCA assay (Thermo Scientific). All antibodies used in this study were obtained from Cell Signaling, except for p100/p52 (Upstate), IκB-ζ (Origene), p105/50 and BCL10 (Santa Cruz), and Tubulin (Sigma-Aldrich).
Enzyme-linked immunosorbent assay (ELISA)
Human interleukin-6 (IL-6) and IL-10 were quantified by ELISA (R&D Systems) according to the manufacturer's instructions.
NF-κB reporter assay
NF-κB activity was quantified using an NF-κB firefly luciferase reporter construct together with a Renilla luciferase vector (phRL-TK) for transient cell transfection. The reporter contained 3 repeats of the NF-κB binding site of IκBα. Cells were lysed in passive lysis buffer (Promega), and luciferase activity was assessed using a dual luciferase assay (Promega) on a TD-20/20 luminometer (Turner Design).
Immunoprecipitation
Immunoprecipitation was performed as described.21 Cell lysates were incubated for 2 hours at 4°C with an affinity-purified anti–IκB-ζ antibody generated using a recombinant N-terminal IκB-ζ fragment comprising amino acids 1 to 400.
Results
IκB-ζ expression is deregulated in ABC DLBCL
To investigate the role of IκB-ζ in the pathogenesis of ABC DLBCL, we assessed IκB-ζ mRNA expression levels in a gene expression dataset of 350 primary DLBCL patient samples.22 We detected high IκB-ζ mRNA expression in ABC DLBCLs, whereas GCB DLBCLs expressed IκB-ζ at significantly lower levels (P = 5.7 × 10−37; Figure 1A). To analyze whether established DLBCL cell lines represent adequate models for functional analyses, we determined IκB-ζ mRNA expression using quantitative PCR. We found that high IκB-ζ expression was restricted to 4 of 5 ABC DLBCL cell lines (HBL-1, OCI-Ly3, OCI-Ly10, and TMD8; Figure 1B). In contrast, the ABC DLBCL cell line U2932, as well as all investigated GCB DLBCL cell lines (HT, BJAB, OCI-Ly1, OCI-Ly2, and OCI-Ly7; Figure 1B), showed low IκB-ζ mRNA levels. Accordingly, IκB-ζ protein expression, as determined by western blotting, was found exclusively in the 4 ABC DLBCL cell lines harboring elevated IκB-ζ mRNA, indicating that increased mRNA levels are translated into protein expression (Figure 1C). As expected, IκB-ζ protein was predominantly localized in the nucleus (supplemental Figure 1).
IκB-ζ is highly expressed in ABC DLBCL and induced by NF-κB. (A) IκB-ζ is significantly higher expressed in ABC compared with GCB DLBCL (P = 5.7 × 10−37). Error bars depict the standard error of the mean. (B) IκB-ζ is highly expressed in 4 ABC DLBCL cell lines. In contrast, in other DLBCL cell lines, only low IκB-ζ expression is detectable by quantitative PCR. Error bars indicate the standard deviation. (C) Western blot analysis of IκB-ζ expression in DLBCL cell lines. Four of 5 ABC DLBCL cell lines have detectable IκB-ζ protein expression. (D) Expression of L265P MYD88 induces IκB-ζ expression in HT and U2932 measured by quantitative PCR and western blotting. (E) Expression of L244P CARD11 induces IκB-ζ expression in HT and U2932 measured by quantitative PCR and western blotting. **P < .01; ***P < .001.
IκB-ζ is highly expressed in ABC DLBCL and induced by NF-κB. (A) IκB-ζ is significantly higher expressed in ABC compared with GCB DLBCL (P = 5.7 × 10−37). Error bars depict the standard error of the mean. (B) IκB-ζ is highly expressed in 4 ABC DLBCL cell lines. In contrast, in other DLBCL cell lines, only low IκB-ζ expression is detectable by quantitative PCR. Error bars indicate the standard deviation. (C) Western blot analysis of IκB-ζ expression in DLBCL cell lines. Four of 5 ABC DLBCL cell lines have detectable IκB-ζ protein expression. (D) Expression of L265P MYD88 induces IκB-ζ expression in HT and U2932 measured by quantitative PCR and western blotting. (E) Expression of L244P CARD11 induces IκB-ζ expression in HT and U2932 measured by quantitative PCR and western blotting. **P < .01; ***P < .001.
Interestingly, western blot analyses of TMD8 protein lysates revealed an IκB-ζ band that migrated slightly faster than expected (approximately 73 kDa; Figure 1C). To identify potential genetic aberrations, we sequenced the coding exons of NFKBIZ in TMD8 cells and identified a heterozygous 159-base-pair in-frame deletion affecting exon 3 (IκB-ζ Δ33-85; supplemental Figure 2A). To characterize the functional consequences of this aberration, we transduced both 293T cells harboring an NF-κB–driven reporter as well as U2932 cells with either wild-type or mutant IκB-ζ. In 293T cells, the NF-κB reporter was activated to a similar degree by wild-type and mutant IκB-ζ (supplemental Figure 2B). Likewise, in U2932 cells, the known IκB-ζ target gene IL-6 was induced at comparable levels by both wild-type and mutant IκB-ζ (supplemental Figure 2C). These data indicate that this internal deletion represents a structural variant that is not associated with gross alterations of protein function, although we cannot exclude differences not detected by our assays. Sequence analysis of the coding exons of NFKBIZ in our panel of DLBCL cell lines did not reveal any other abnormalities involving NFKBIZ (data not shown). Likewise, analysis of recently published sequencing studies of primary DLBCL patient samples26,27 did not reveal any aberrations involving NFKBIZ, indicating that NFKBIZ is not recurrently targeted by somatic mutations.
Constitutive NF-κB signaling upregulates IκB-ζ in ABC DLBCL
We performed aCGH in our cell line panel to elucidate molecular mechanisms that account for high IκB-ζ expression. However, these analyses did not reveal amplifications of the NFKBIZ locus in any ABC or GCB DLBCL cell line (data not shown).
Expression of several IκBs is regulated by NF-κB. To assess the effect of NF-κB signaling on IκB-ζ expression in ABC DLBCL, we expressed mutant or wild-type MYD88 and CARD11 proteins in DLBCL cell lines that are devoid of IκB-ζ expression. MYD88 functions as a signaling adaptor molecule that activates NF-κB after stimulation of Toll-like receptors by recruiting IRAK kinases.28 The L265P MYD88 mutation is detectable in roughly 30% of ABC DLBCL patient samples and promotes constitutive NF-κB signaling.12 Transduction of HT and U2932 cells with the L265P mutant significantly induced IκB-ζ mRNA and protein expression (Figure 1D). Conversely, shRNA-mediated knockdown of MYD88 in the ABC DLBCL cell lines HBL-1 and OCI-Ly3, which both harbor the L265P MYD88 mutation,12 significantly downregulated IκB-ζ mRNA and protein levels (supplemental Figure 3). In a similar fashion, we expressed the L244P CARD11 mutant in HT and U2932. CARD11 mutations occur in 10% of ABC DLBCLs and mediate constitutive NF-κB activation by assembling a signaling complex with BCL10 and MALT1.10 The L244P CARD11 mutant induced IκB-ζ expression to a comparable degree as the L265P MYD88 mutant (Figure 1E), indicating that constitutive NF-κB signaling drives IκB-ζ expression in ABC DLBCL.
Downregulation of IκB-ζ is selectively toxic to ABC DLBCL
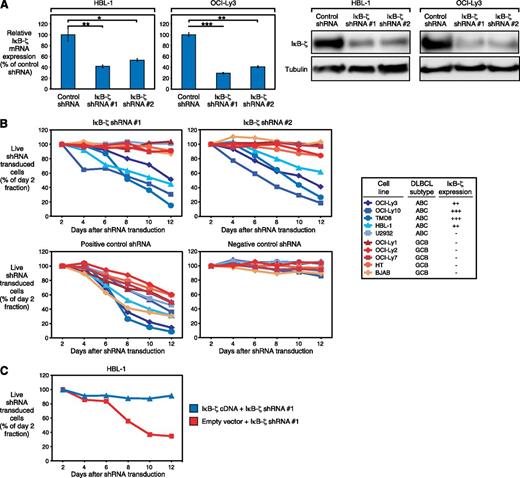
To elucidate the functional significance of IκB-ζ in ABC DLBCL, we knocked down its expression, using specific shRNAs. We identified 2 different IκB-ζ shRNAs that significantly decreased IκB-ζ expression on both the mRNA and protein levels (Figure 2A). Transduction of these shRNAs induced toxicity in IκB-ζ-positive ABC DLBCL cell lines (HBL-1, OCI-Ly3, OCI-Ly10, and TMD8). In contrast, these shRNAs did not affect survival of IκB-ζ-negative ABC (U2932) or GCB DLBCLs (HT, BJAB, OCI-Ly1, OCI-Ly2, and OCI-Ly7), whereas positive control shRNAs (directed against CARD11 in ABC DLBCL and MYC in GCB DLBCL cell lines) were toxic to all cell lines (Figure 2B). To demonstrate that IκB-ζ shRNA-mediated toxicity was caused by IκB-ζ knockdown, we transduced the IκB-ζ coding region in HBL-1 cells to perform a rescue experiment. Indeed, exogenous IκB-ζ expression rescued HBL-1 cells from shRNA-mediated toxicity, indicating the specificity of our approach (Figure 2C).
Knockdown of IκB-ζ is toxic to ABC DLBCL. (A) IκB-ζ shRNA 1 and 2 downregulate IκB-ζ mRNA and protein in HBL-1 and OCI-Ly3, measured by quantitative PCR and western blotting. Error bars indicate the standard deviation. (B) IκB-ζ knockdown by 2 independent shRNAs is toxic to IκB-ζ-positive ABC DLBCL cell lines. In contrast, IκB-ζ-negative DLBCLs are not affected by IκB-ζ downregulation. Positive control shRNAs directed against CARD11 (for ABC DLBCL cell lines) or MYC (for GCB DLBCL cell lines) were toxic to all DLBCLs, whereas a negative control shRNA against MSMO1 is not toxic to any cell line. Representative results from at least 3 independent replicates are shown. Baseline expression of IκB-ζ in the investigated cell lines is indicated, based on western blotting (Figure 1C). (C) Exogenous expression of IκB-ζ cDNA rescues HBL-1 cells from IκB-ζ shRNA-mediated toxicity. Representative results from 3 independent replicates are shown. *P < .05; **P < .01; ***P < .001.
Knockdown of IκB-ζ is toxic to ABC DLBCL. (A) IκB-ζ shRNA 1 and 2 downregulate IκB-ζ mRNA and protein in HBL-1 and OCI-Ly3, measured by quantitative PCR and western blotting. Error bars indicate the standard deviation. (B) IκB-ζ knockdown by 2 independent shRNAs is toxic to IκB-ζ-positive ABC DLBCL cell lines. In contrast, IκB-ζ-negative DLBCLs are not affected by IκB-ζ downregulation. Positive control shRNAs directed against CARD11 (for ABC DLBCL cell lines) or MYC (for GCB DLBCL cell lines) were toxic to all DLBCLs, whereas a negative control shRNA against MSMO1 is not toxic to any cell line. Representative results from at least 3 independent replicates are shown. Baseline expression of IκB-ζ in the investigated cell lines is indicated, based on western blotting (Figure 1C). (C) Exogenous expression of IκB-ζ cDNA rescues HBL-1 cells from IκB-ζ shRNA-mediated toxicity. Representative results from 3 independent replicates are shown. *P < .05; **P < .01; ***P < .001.
IκB-ζ is required for nuclear NF-κB activity in ABC DLBCL
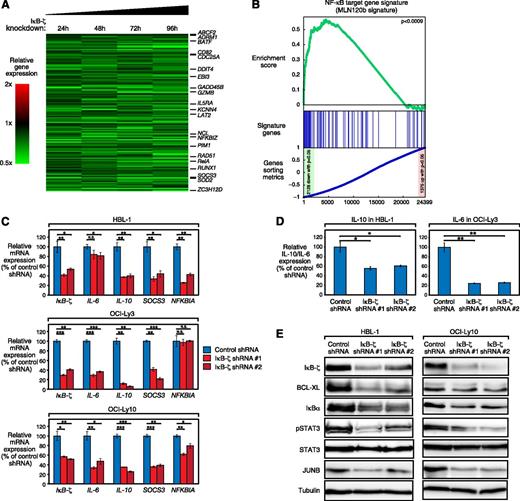
To obtain additional insights into which biologic processes are regulated by IκB-ζ in ABC DLBCL, we profiled gene expression changes after IκB-ζ knockdown in HBL-1 cells. We identified 88 genes that were significantly upregulated (P < .0025) and 136 genes that were downregulated (P < .0025) across all times after IκB-ζ knockdown (Figure 3A; supplemental Table 1). A large number of genes affected by IκB-ζ knockdown were either known NF-κB targets or involved in cellular processes such as inflammation or immune response (eg, SOCS3, BATF, BCL3, CDC25A, IL5RA, and EBI3). These results suggest that IκB-ζ downregulation deregulates NF-κB activity. To test this hypothesis, we performed an unbiased gene set enrichment analysis, using previously described gene expression signatures.29 This signature database was complemented by 2 target gene signatures that were defined by treatment of HBL-1 cells with specific inhibitors against either IKKβ (MLN120b) or MALT1 (z-VRPR-fmk)30 (supplemental Figure 4A-B; supplemental Tables 2 and 3). Gene set enrichment analysis revealed a significant enrichment of IκB-ζ targets in both the IKKβ inhibitor–defined (P < .0009; Figure 3B) and MALT1 inhibitor–defined (P < .001; supplemental Figure 4C) signatures, as well as in 2 additional NF-κB target gene signatures (NFκB_Up_B-cell-receptor_paper [P < .002] and NFκB_Up_bothOCILy3andLy10 [P < .02]; supplemental Table 4). Conversely, genes that were upregulated after IκB-ζ knockdown were significantly enriched in NF-κB gene signatures that encompass upregulated target genes after NF-κB inhibition (supplemental Table 4). These results demonstrate that IκB-ζ is essential for nuclear NF-κB activity in ABC DLBCL. Interestingly, IκB-ζ target genes were also enriched in a gene set that distinguishes ABC from other lymphoma subtypes, suggesting that the IκB-ζ gene signature is expressed at higher levels in primary ABC DLBCL patient samples compared with other malignant lymphoma subtypes (supplemental Figure 4D; supplemental Table 4).
IκB-ζ downregulation deregulates NF-κB activity in ABC DLBCL. (A) Gene expression profiling after IκB-ζ knockdown in HBL-1 cells. Changes of gene expression were profiled at the indicated time points after induction of IκB-ζ shRNA 1. Gene expression changes are depicted according to the color scale shown. Known NF-κB target genes and genes involved in processes such as inflammation are highlighted. (B) Gene set enrichment analysis of the MLN120b-defined NF-κB gene expression signature. The NF-κB signature is significantly downregulated after IκB-ζ knockdown. (C) IκB-ζ knockdown downregulates NF-κB target genes in HBL-1, OCI-Ly3, and OCI-Ly10, as measured by quantitative PCR. Error bars indicate the standard deviation. (D) IκB-ζ knockdown downregulates IL-10 in IL-10-expressing HBL-1 and IL-6 in IL-6-expressing OCI-Ly3 cells measured by ELISA. Error bars indicate the standard deviation. (E) IκB-ζ knockdown downregulates NF-κB targets in HBL-1 and OCI-Ly10, as measured by western blotting. N.S., not significant; *P < .05; **P < .01; ***P < .001.
IκB-ζ downregulation deregulates NF-κB activity in ABC DLBCL. (A) Gene expression profiling after IκB-ζ knockdown in HBL-1 cells. Changes of gene expression were profiled at the indicated time points after induction of IκB-ζ shRNA 1. Gene expression changes are depicted according to the color scale shown. Known NF-κB target genes and genes involved in processes such as inflammation are highlighted. (B) Gene set enrichment analysis of the MLN120b-defined NF-κB gene expression signature. The NF-κB signature is significantly downregulated after IκB-ζ knockdown. (C) IκB-ζ knockdown downregulates NF-κB target genes in HBL-1, OCI-Ly3, and OCI-Ly10, as measured by quantitative PCR. Error bars indicate the standard deviation. (D) IκB-ζ knockdown downregulates IL-10 in IL-10-expressing HBL-1 and IL-6 in IL-6-expressing OCI-Ly3 cells measured by ELISA. Error bars indicate the standard deviation. (E) IκB-ζ knockdown downregulates NF-κB targets in HBL-1 and OCI-Ly10, as measured by western blotting. N.S., not significant; *P < .05; **P < .01; ***P < .001.
To confirm the role of IκB-ζ for NF-κB activity in ABC DLBCL, we determined the expression of several well-characterized NF-κB target genes in additional cell lines by quantitative PCR. With the exception of NFKBIA in OCI-Ly3, we observed a significant downregulation of all investigated NF-κB targets after IκB-ζ knockdown (Figure 3C). In addition, we analyzed whether IκB-ζ knockdown leads to downregulation of known NF-κB targets on protein level. To this end, we determined IL-6 and IL-10 expression in the supernatant of OCI-Ly3 and HBL-1 cells by ELISA (Figure 3D), as well as expression of BCL-XL, JUNB, IκBα, and pSTAT3 by western blotting in HBL-1 and OCI-Ly10 (Figure 3E). These analyses revealed that all investigated targets were downregulated after IκB-ζ knockdown (Figure 3D-E), suggesting that IκB-ζ plays a central role in regulating NF-κB activity in ABC DLBCL.
IκB-ζ interacts with NF-κB subunits p50 and p52
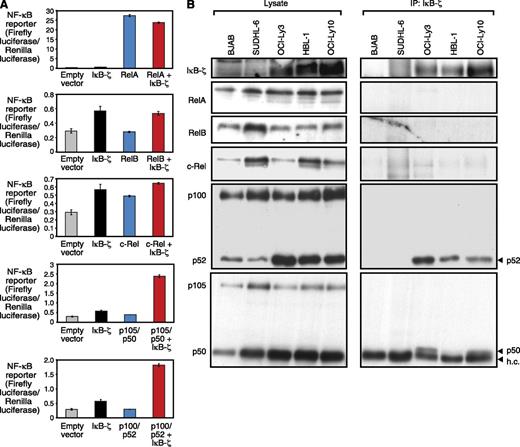
Previous work suggested that IκB-ζ binds to NF-κB subunits p50 and p65 to repress or induce expression of NF-κB target genes.31,32 To identify IκB-ζ interaction partners in ABC DLBCL, we initially used 293T cells to investigate the interaction of IκB-ζ with individual NF-κB subunits. To this end, we transfected 293T cells with IκB-ζ and individual NF-κB subunits and measured the activity of an NF-κB reporter construct (Figure 4A). As expected, we found a functional interaction between IκB-ζ and p50, as measured by an increase in NF-κB reporter activity that was not observed with IκB-ζ or p50 alone. In contrast, no functional interaction between IκB-ζ and RelA, RelB, or c-Rel was detectable. However, we also observed a previously unappreciated interaction between IκB-ζ and p52 (Figure 4A). To validate these findings, we performed IκB-ζ coimmunoprecipitation assays in DLBCL cell lines. These analyses confirmed a physical interaction of IκB-ζ with p50 and p52 in ABC but not GCB DLBCL cell lines (Figure 4B). The detected p50 band in HBL-1 was weak (Figure 4B), most likely because of low nuclear p50 expression in these cells (supplemental Figure 4E). In contrast, no interaction of IκB-ζ with RelA, RelB, or c-Rel was detectable.
IκB-ζ functionally interacts with the NF-κB subunits p50 and p52. (A) NF-κB reporter activity after co-transfection of individual NF-κB subunits and IκB-ζ measured in 293T cells. IκB-ζ synergizes with p50 and p52, but not with RelA, RelB, or c-Rel. (B) IκB-ζ coimmunoprecipitation assays show a physical interaction of IκB-ζ with p50 and p52 in the ABC DLBCL cell lines OCI-Ly3, OCI-Ly10, and HBL-1. h.c., heavy chain.
IκB-ζ functionally interacts with the NF-κB subunits p50 and p52. (A) NF-κB reporter activity after co-transfection of individual NF-κB subunits and IκB-ζ measured in 293T cells. IκB-ζ synergizes with p50 and p52, but not with RelA, RelB, or c-Rel. (B) IκB-ζ coimmunoprecipitation assays show a physical interaction of IκB-ζ with p50 and p52 in the ABC DLBCL cell lines OCI-Ly3, OCI-Ly10, and HBL-1. h.c., heavy chain.
Other NF-κB–dependent lymphoma subtypes are not addicted to IκB-ζ signaling
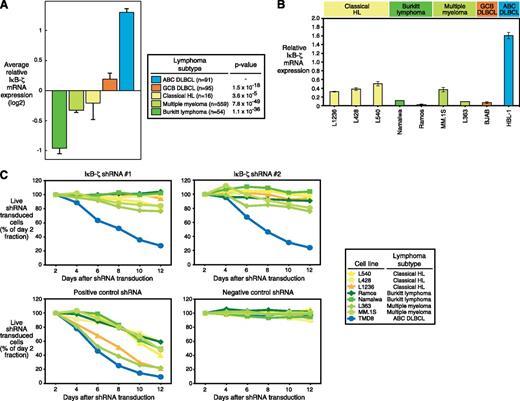
To elucidate whether IκB-ζ is involved in the biology of other malignant lymphoma subtypes, we assessed IκB-ζ mRNA expression levels in 815 primary patient samples from NF-κB–dependent (ABC DLBCL, cHL, and MM) and independent entities (BL and GCB DLBCL).23-25 High IκB-ζ expression was only detectable in ABC DLBCL. In contrast, BL, GCB DLBCL, cHL, and MM patient samples had low IκB-ζ expression compared with ABC DLBCL (Figure 5A). Next, we used various cell line models of these subtypes to functionally investigate the role of IκB-ζ in the pathogenesis of these entities. Quantitative PCR revealed that these lines express low levels of IκB-ζ (Figure 5B), consistent with the finding in the primary patient samples. As expected, shRNA-mediated IκB-ζ knockdown was not toxic to any of the investigated cHL, MM, or BL cell lines (Figure 5C). In contrast, a positive control shRNA directed against MYC was toxic to all cell lines. Collectively, these data indicate that IκB-ζ is not involved in the molecular pathogenesis of cHL, MM, or BL.
Other NF-κB–dependent lymphoma subtypes are not addicted to IκB-ζ signaling. (A) ABC DLBCLs express higher IκB-ζ mRNA levels compared with other NF-κB–dependent and independent lymphoma subtypes. Error bars depict the standard error of the mean. (B) IκB-ζ is highly expressed in the ABC DLBCL cell line HBL-1. In contrast, in cHL, BL, MM, and GCB DLBCL cell lines, only low IκB-ζ expression is detectable by quantitative PCR. Error bars indicate the standard deviation. (C) Knockdown of IκB-ζ by 2 independent shRNAs is not toxic to BL, cHL, and MM cell lines. A positive control shRNA directed against MYC is toxic to all cell lines, whereas a negative control shRNA against MSMO1 is not toxic to any cell line. Representative results from at least 3 independent replicates are shown.
Other NF-κB–dependent lymphoma subtypes are not addicted to IκB-ζ signaling. (A) ABC DLBCLs express higher IκB-ζ mRNA levels compared with other NF-κB–dependent and independent lymphoma subtypes. Error bars depict the standard error of the mean. (B) IκB-ζ is highly expressed in the ABC DLBCL cell line HBL-1. In contrast, in cHL, BL, MM, and GCB DLBCL cell lines, only low IκB-ζ expression is detectable by quantitative PCR. Error bars indicate the standard deviation. (C) Knockdown of IκB-ζ by 2 independent shRNAs is not toxic to BL, cHL, and MM cell lines. A positive control shRNA directed against MYC is toxic to all cell lines, whereas a negative control shRNA against MSMO1 is not toxic to any cell line. Representative results from at least 3 independent replicates are shown.
Discussion
Within the present study, we have identified an unexpected role of the atypical, nuclear IκB family member IκB-ζ14-16 in the biology of ABC DLBCL. Constitutive activation of the oncogenic NF-κB pathway is a characteristic hallmark of this lymphoma subtype.7 Recently, various somatically acquired mutations affecting positive (eg, CARD11, CD79A, CD79B, and MYD88) and negative (eg, TNFAIP3 [A20]) NF-κB regulators4,10-13 have been identified that cause aberrant activation of this signaling cascade in ABC DLBCL. However, our understanding of how specific NF-κB target genes are regulated is still rudimentary. Here, we provide molecular insights that IκB-ζ is essential for the expression of a specific set of NF-κB target genes in ABC DLBCL.
Our gene expression profiling analyses demonstrate that IκB-ζ regulates the expression of a large number of known NF-κB target genes by physical interaction with the NF-κB subunits p50 and p52, respectively. This finding was unexpected, as previous studies have shown that although IκB-ζ can induce expression of individual NF-κB targets such as IL-6, it can also act as a repressor of NF-κB signaling.14,19 The interaction of IκB-ζ with both p50 and p52 was also unanticipated, as previous work has suggested that IκB-ζ interacts with either p50 or p65.31,32 The interaction of IκB-ζ with p52 has not been recognized before, and therefore our data indicate that IκB-ζ interacts with components of both the canonical and the noncanonical NF-κB pathway in ABC DLBCL. Fittingly, activation of both p50 and p52 in these lymphomas, and in some instances in the same sample, has previously been shown by immunohistochemical staining of nuclear p50 and p52 expression.4 Engagement of the noncanonical pathway leads preferentially to the accumulation of p52/RelB heterodimers.9 Recent work has suggested that constitutive MALT1 activity cleaves RelB that is subsequently degraded in the proteasome.33 It appears conceivable that MALT1-induced RelB cleavage might facilitate an interaction between p52 and IκB-ζ.
The importance of IκB-ζ for ABC DLBCL biology is further underscored, as its shRNA-mediated knockdown induced toxicity in ABC DLBCL models, suggesting that the IκB-ζ–controlled target genes are essential for ABC DLBCL survival. Thus, our results indicate that IκB-ζ is an essential mediator of NF-κB activity in ABC DLBCL.
IκB-ζ expression seems to be controlled through NF-κB signaling in ABC DLBCL in the vast majority of cases, as its expression was induced by mutants identified in patient samples that activate the NF-κB pathway. Thus, the primary mode of IκB-ζ upregulation in primary ABC DLBCL patient samples seems to be the constitutive activation of NF-κB signaling that is detectable in the vast majority of cases.3,4 Interestingly, some ABC DLBCL samples such as the U2932 cell line do not have detectable IκB-ζ expression levels, despite being characterized by constitutive NF-κB signaling. The underlying molecular mechanisms for this phenomenon remain unknown but could be related to differences in mRNA stabilization.34
The contribution of the previously identified amplifications of the NFKBIZ locus20 to aberrant IκB-ζ expression remains unclear, as these abnormalities are detectable in only 10% of ABC DLBCL cases. However, these cases are presumably also characterized by constitutive NF-κB signaling. Intriguingly, other NF-κB–dependent lymphoma subtypes such as cHL or MM35-37 do not express high IκB-ζ levels, and fittingly, their survival is not affected by IκB-ζ knockdown. This finding of a predominant expression pattern in ABC DLBCL was confirmed by gene set enrichment analyses, which showed that the IκB-ζ target gene signature was enriched in a gene set that distinguishes ABC from other lymphoma subtypes, suggesting that these target genes are indeed expressed at higher levels in primary ABC DLBCL patient samples compared with other malignant lymphoma subtypes. Collectively, these results indicate that expression of IκB-ζ, despite being an NF-κB target gene, is context-dependent and potentially regulated by unknown additional molecular events that are ABC DLBCL-specific.
Finally, our data suggest that IκB-ζ, despite the fact that it might be difficult to target directly, represents a promising therapeutic molecular target for the treatment of ABC DLBCL. Patients with ABC DLBCL are characterized by adverse survival after conventional standard treatment with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone.22 All ABC DLBCL models with detectable IκB-ζ expression were sensitive to IκB-ζ knockdown, suggesting that inhibition of components that mediate IκB-ζ expression might offer a novel therapeutic strategy for patients diagnosed with ABC DLBCL.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants from the Deutsche Krebshilfe, the German Research Foundation, and the Else Kröner-Fresenius-Stiftung (G.L.), a doctoral scholarship from the Philipps-University Marburg (M.G.), and research grants from the Swiss National Science Foundation and the Swiss Cancer League (Oncosuisse) (M.T.).
Authorship
Contribution: H.N. and S.-S.W. designed research, performed experiments, and wrote the manuscript; S.H. performed and analyzed experiments; M.G. performed bioinformatic and biophysical analyses; E.K., V.S., B.W.-W., M.P., A.W., M.F., K.D., and H.M. performed and analyzed experiments; A.T., M.H., B.D., C.S., M.J., and M.T. analyzed data; P.L. performed and supervised bioinformatic and biophysical analyses; and G.L. designed research, analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georg Lenz, Department of Hematology, Oncology, and Tumor Immunology, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: georg.lenz@charite.de.
References
Author notes
H.N. and S.-S.W. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal