Key Points
HSPCs are recruited to S aureus–infected skin wounds, differentiate into neutrophils, and hasten resolution of infection.
Bacterial sensing via TLR2 elicits PGE2 production in HSPCs that provides autocrine feedback to meet the demand for local granulopoiesis.
Abstract
During bacterial infection, hematopoietic stem and progenitor cells (HSPCs) differentiate into polymorphonuclear leukocytes (PMNs) in the bone marrow. We reported that HSPCs recruited to Staphylococcus aureus–infected skin wounds in mice undergo granulopoiesis, whereas other authors have demonstrated their differentiation in vitro after Toll-like receptor 2 (TLR2)/MyD88 stimulation. Here, we examined this pathway in HSPC trafficking and granulopoiesis within S aureus–infected wounds. Lineage− HSPCs from TLR2- or MyD88-deficient mice injected into infected wounds of wild-type (WT) mice exhibited impaired granulopoiesis. However, HSPCs from WT mice produced similar numbers of PMNs whether transferred into wounds of TLR2-, MyD88-deficient, or WT mice. Prostaglandin E2 (PGE2), which stimulates HSPC survival and proliferation, was produced by HSPCs after TLR2 stimulation, suggesting that TLR2/MyD88 activation promotes granulopoiesis in part by production and autocrine activity of PGE2. Pretreatment of TLR2- or MyD88-deficient HSPCs with PGE2 rescued granulocytic differentiation in vivo. Finally, we demonstrate that bone marrow–derived lin−/Sca-1+/c-kit+ cells produced PGE2 and underwent granulopoiesis after TLR2 stimulation. lin−/Sca-1+/c-kit+ cells deficient in TLR2 or MyD88 produced PMNs after PGE2 treatment when transferred into uninfected wounds. We conclude that granulopoiesis in S aureus–infected wounds is induced by TLR2/MyD88 activation of HSPCs through a mechanism that involves autocrine production and activity of PGE2.
Introduction
Polymorphonuclear cells (PMNs) are first-line effector cells of the innate immune system recruited to a bacterial nidus. They are produced in the bone marrow (BM) by hematopoietic stem and progenitor cells (HSPCs), which undergo compensatory granulopoiesis when mature PMNs rapidly exit BM to traffic to the site of bacterial infection.1 However, mounting evidence suggests that HSPCs themselves may contribute to the innate immune response. HSPCs consist of a heterogeneous population of cells, which include rare quiescent hematopoietic stem cells (HSCs), and larger numbers of multipotent and highly proliferative early progenitor cells that express high levels of c-kit and Sca-1 and are the source of common myeloid progenitors (CMPs), which possess more limited proliferative capacity and expand to phagocytes. Inflammation elicits HSPC mobilization from BM and recruitment to sites of tissue injury,2-4 prompting the current study on the role of HSPCs in the innate immune response to cutaneous infection.
HSPCs traffic to Staphylococcus aureus–infected skin wounds where they differentiate to PMNs.3 Although the majority of PMNs enter wounds from the circulation as mature cells, we recently reported that up to 20% are derived locally from HSPCs and contribute to host defense and bacterial clearance.3 HSPCs collected from S aureus–infected wounds formed threefold more granulocyte colonies than those harvested from uninfected wounds,3 suggesting that the granulopoietic potential of HSPCs is responsive to signals encountered in the presence of an infection. Previous studies have shown that HSPCs can detect and respond to bacteria through Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns.5 In particular, TLR2 is activated by S aureus lipopeptides, lipoteichoic acid and peptidoglycan.6 Triggering TLR2 on HSPCs leads to signaling via MyD88, the common adaptor protein used by all TLRs (except TLR3), which activates HSPC proliferation and myeloid differentiation in the absence of hematopoietic growth factors in vitro.5,7 However, TLR signaling is not required for HSPC expansion in the BM during a systemic S aureus infection,8 and whether direct microbial activation of HSPCs occurs in vivo is unknown.
HSPCs express all known prostaglandin E2 (PGE2) receptors, and exogenous PGE2 enhances HSPC survival and entry into cell cycle after ex vivo incubation.9 PGE2 is rapidly metabolized in vivo by prostaglandin dehydrogenase and may act in an autocrine or paracrine manner to activate transcription and control posttranscriptional gene expression.10,11 For instance, macrophages produce PGE2 after TLR2 activation,12 but it is unknown if HSPCs function equivalently. Thus, one potential mechanism for expansion of HSPCs after TLR2 activation is by production of PGE2 by resident macrophages.12 Alternatively, HSPC themselves may elaborate PGE2 as a downstream mediator of TLR2 activation providing a mechanism for inducing HSPC differentiation to PMNs within the wound abscess.
In this study, we demonstrate the role of HSPCs in promoting an effective immune response against a S aureus–infected skin wound. During skin wounding, an initial efflux of HSPCs is induced from the BM, which are recruited to the wound and expand their numbers to produce mature PMNs. However, in S aureus–infected wounds, increased numbers of HSPCs traffic to the wound and produce PMNs. BM cells enriched for HSPCs by lineage depletion from TLR2- or MyD88-deficient mice had severely impaired granulopoiesis at the site of infection, suggesting HSPCs are directly activated by the S aureus infection in the wound rather than by wound inflammation per se. Pretreatment of HSPCs or BM cells enriched for HSCs (lineage-negative/c-kit+/Sca-1+; LSK cells) from TLR2- or MyD88-deficient mice with PGE2 restored granulopoiesis in vivo to levels detected in wild-type (WT) mice. These data demonstrate that HSPCs, including oligopotent LSK cells, undergo granulopoiesis at the site of a bacterial infection in the periphery through direct activation of TLR2/MyD88 and the activity of PGE2 to promote optimal host defense and bacterial clearance.
Materials and methods
Mice
Male and female mice 8 to 16 weeks old on a C57BL/6 genetic background were used in all of the experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California at Davis and were performed following the guidelines of the Animal Welfare Act and Health Research Extension Act.
Mouse model of S aureus wound infection
Skin wounds were created and inoculated with S aureus as described previously13 and in supplemental Methods.
Preparation of bioluminescent S aureus and noninvasive quantification of wound EGFP-PMN and bioluminescent bacteria
S aureus was cultured and prepared as described previously13 and in supplemental Methods. Actively metabolizing bacteria and enhanced green fluorescent protein (EGFP)-PMN fluorescence at wound sites over time were quantified using the Xenogen IVIS 100 imaging system and Living Image 2.5 software (Caliper Life Science), as described previously.3,13
Immunodepletion of c-kit+ HSPC
HSPC depletion was performed as described previously3 and in supplemental Methods.
In vitro proliferation of HSPCs
A total of 500 000 BM HSPCs per condition, enriched by lineage depletion using magnetic beads (Stem Cell Technologies), were incubated at 37°C with heat-killed (95°C for 5 minutes) S aureus (1 × 108/mL) with or without indomethacin (0.5 μM; Sigma), PGE2 (10 μg/mL; Cayman Chemical), or no stimulus.
Adoptive transfer of HSPCs
BM HSPCs from unwounded mice were enriched by lineage depletion (Stem Cell Technologies) to provide a cell solution that was ∼4% LSK cells, 17% other lin−/c-kit+ progenitors, and only 0.3% PMNs. In some experiments, a more pure population was required, and lin−/Sca-1+/c-kit+ cells (LSKs) were sorted via the fluorescence-activated cell sorter (FACS) after bead enrichment by lineage depletion.
Statistical analysis
Data analysis was performed using GraphPad Prism version 5.0 (GraphPad Software).
Results
HSPCs contribute to the innate immune response in S aureus–infected wounds
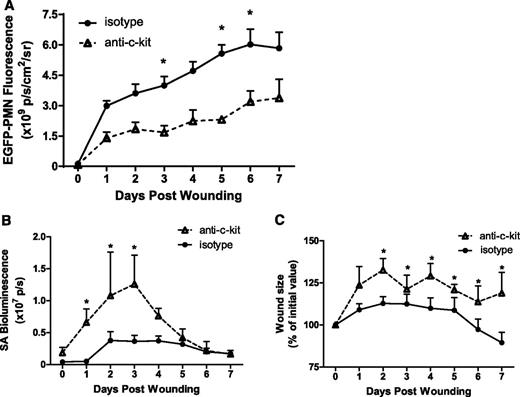
HSPCs traffic to skin wounds where they contribute to the innate immune response to wounding and S aureus infection by differentiating into mature PMNs.3 The physiological significance of HSPCs to this response was examined by systemic antibody depletion of HSPCs using the anti-c-kit antibody ACK2. We previously demonstrated that antibody depletion resulted in a 60% decrease in BM HSPCs leading to a 30% reduction of BM PMN numbers.3 Here, we set out to determine the impact of depletion of HSPCs on the cellular composition in S aureus–infected skin wounds. After antibody depletion, wound PMN numbers in HSPC-depleted mice were decreased 42% to 50% at 7 days, a much larger reduction in PMN number than can be explained by antibody-mediated BM depletion alone (Figure 1A). Taking into account the 30% reduction in BM PMNs available for trafficking to the wound after treatment, we estimate that 28% of the PMNs in the wound were derived by local HSPC differentiation. Circulating numbers of PMNs or other mononuclear leukocytes were not statistically altered by treatment with anti-c-kit (supplemental Figure 2). Depletion of this population of wound PMNs negatively affected clearance of bacteria and delayed wound healing. It correlated with a twofold increase in the bacterial burden in the wound on days 1 to 3 after inoculation (Figure 1B), which was accompanied by a ∼30% increase in wound size compared with controls (Figure 1C). These data indicate that HSPC-expanded PMNs play a central role in bacterial clearance and wound closure.
HSPCs contribute to the innate immune response during cutaneous S aureus infection. Lys-EGFP mice were treated with a mAb to c-kit to deplete BM HSPC numbers, or to an isotype control antibody. Full-thickness skin wounds 6 mm in diameter were inoculated with 1 × 107 CFUs of a bioluminescent S aureus strain. (A) Kinetics of EGFP-PMN fluorescence after wounding and inoculation at day 0. (B) S aureus bioluminescent signal and (C) wound size as the percentage of day 0 value all measured using whole animal fluorescence imaging of live animals for 7 days after wounding. Data represent 5 to 8 mice per group and are expressed as mean ± standard error of the mean (SEM) (*P < .05).
HSPCs contribute to the innate immune response during cutaneous S aureus infection. Lys-EGFP mice were treated with a mAb to c-kit to deplete BM HSPC numbers, or to an isotype control antibody. Full-thickness skin wounds 6 mm in diameter were inoculated with 1 × 107 CFUs of a bioluminescent S aureus strain. (A) Kinetics of EGFP-PMN fluorescence after wounding and inoculation at day 0. (B) S aureus bioluminescent signal and (C) wound size as the percentage of day 0 value all measured using whole animal fluorescence imaging of live animals for 7 days after wounding. Data represent 5 to 8 mice per group and are expressed as mean ± standard error of the mean (SEM) (*P < .05).
HSPC differentiation in response to S aureus is regulated locally in skin wounds
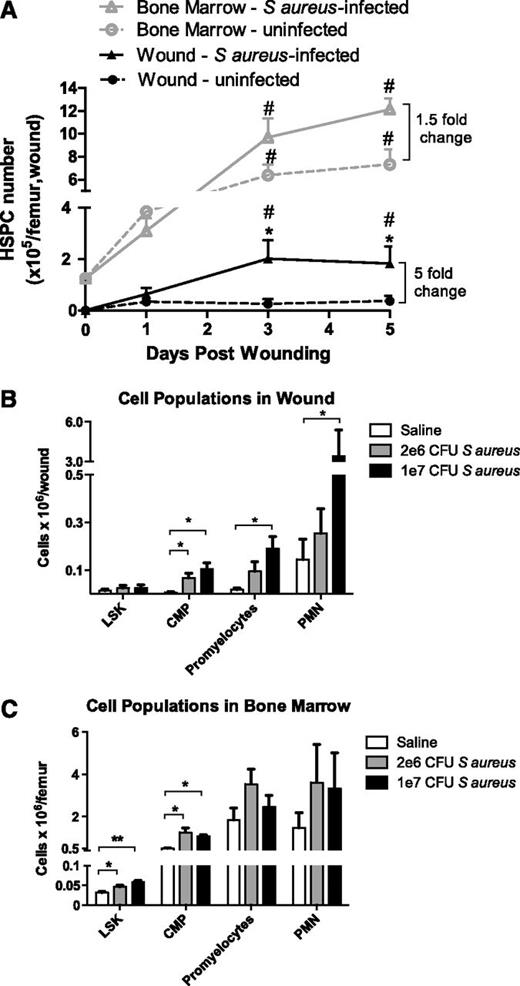
HSPC numbers, identified by flow cytometry as lin−/c-kit+ cells, increase significantly in the BM from days 3 to 5 after wounding with slightly elevated levels in response to S aureus infection (Figure 2A). In contrast, HSPC numbers in the wound significantly increased in S aureus–inoculated wounds compared with saline-treated wounds. Remarkably, the number of HSPCs detected in the abscess at day 3 was 50% greater compared with their numbers in the BM of a femur at baseline. Immunophenotypic analysis of granulocytic progenitors in the BM and wound (supplemental Figure 1) revealed an early and rapid efflux of LSK cells (a lin−/ c-kit+/Sca-1+ population enriched for HSCs), CMPs, and promyelocytes from the BM that coincided with the kinetics of influx of these populations into the abscess (supplemental Figures 3 and 4). The number of CMPs, promyelocytes, and PMNs within the wound increased significantly with time, whereas LSK cell numbers returned to baseline by day 5 (supplemental Figure 3). A dose-dependent effect of S aureus on the number of myeloid progenitors and their expansion into PMNs was observed in the abscess, which was absent in the BM (Figure 2B-C). By day 3, promyelocyte and PMN populations in the BM were no longer significantly different between S aureus–inoculated vs saline-treated wounds. In contrast, CMPs and PMNs on day 5 were significantly elevated in S aureus–inoculated wounds compared with saline-treated wounds (supplemental Figure 3). These data indicate that infection elicited a greater effect on the number of myeloid-committed cells and their progeny in the abscess than in the BM.
Numbers of HSPCs and their progeny in BM and cutaneous wounds infected with S aureus. (A) Full-thickness skin wounds (6 mm) were created on the backs of WT mice and were inoculated with 1 × 107 CFUs of S aureus (triangles) or saline vehicle control (circles). Lineage-negative, c-kit+ HSPCs in BM and skin wound digests were evaluated via flow cytometry on days 1, 3, and 5 after wounding. (# indicates significant difference compared with day 0, P < .05; *significant difference compared with saline, P < .05) (B-C) Populations of PMNs and their progenitors in skin wounds (B) and BM (C) of WT mice were measured via flow cytometry 3 days after wounding. LSK refers to lineage-negative, Sca-1+, c-kit+ cells, a population enriched for hematopoietic stem cells; CMPs, common myeloid progenitor cells; bands and PMNs, immature banded and mature segmented neutrophils. (**significant difference compared with saline, P < .01) Data represent 8 mice per group during 3 experiments and are expressed as mean ± SEM.
Numbers of HSPCs and their progeny in BM and cutaneous wounds infected with S aureus. (A) Full-thickness skin wounds (6 mm) were created on the backs of WT mice and were inoculated with 1 × 107 CFUs of S aureus (triangles) or saline vehicle control (circles). Lineage-negative, c-kit+ HSPCs in BM and skin wound digests were evaluated via flow cytometry on days 1, 3, and 5 after wounding. (# indicates significant difference compared with day 0, P < .05; *significant difference compared with saline, P < .05) (B-C) Populations of PMNs and their progenitors in skin wounds (B) and BM (C) of WT mice were measured via flow cytometry 3 days after wounding. LSK refers to lineage-negative, Sca-1+, c-kit+ cells, a population enriched for hematopoietic stem cells; CMPs, common myeloid progenitor cells; bands and PMNs, immature banded and mature segmented neutrophils. (**significant difference compared with saline, P < .01) Data represent 8 mice per group during 3 experiments and are expressed as mean ± SEM.
TLR2 and MyD88 are essential for HSPC granulocytic differentiation in S aureus–inoculated skin wounds
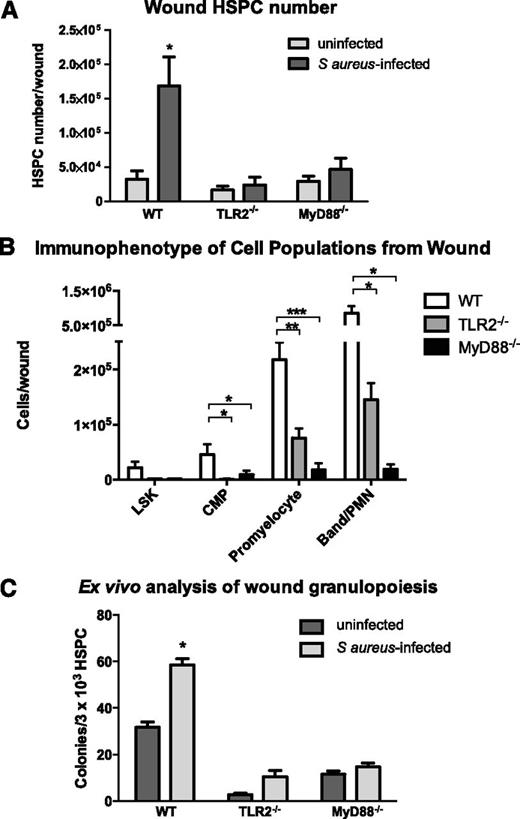
The adaptor molecule MyD88 is necessary for optimal recruitment of PMNs to S aureus–infected wounds where it mediates intracellular signaling in response to activation of TLR and IL-1R family members.14-16 Because HSPCs express TLRs on their surface,5 we examined the role of MyD88 and TLR2 in HSPC proliferation and granulocytic differentiation after wounding and infection. We first examined signaling of myeloid expansion in LSK cells from WT mice. A dose-dependent effect of the TLR2 agonist Pam3CSK4 on PMN production was observed at 96 hours of ex vivo culture of LSK cells. At day 7, this resulted in a 50-fold expansion that was specific for myeloid cell production dependent on TLR2 signaling (supplemental Figure 5). Mice lacking MyD88 or TLR2 exhibited normal numbers of HSPCs in BM and peripheral blood (supplemental Figure 6). In contrast, LSK cell numbers in S aureus–inoculated wounds of TLR2- or MyD88-deficient mice trended lower but were not significantly different from WT mice. However, far fewer of their progeny including CMPs, promyelocytes, and PMNs were detected in the abscess (Figure 3B).
TLR2- or MyD88-deficient mice exhibit diminished granulocyte differentiation in S aureus–infected wounds. Wounds from WT mice, TLR2-deficient, and MyD88-deficient mice were collected 3 days after wounding (+/−S aureus inoculation). (A) Total lineage-negative/c-kit+ HSPCs isolated from wounds of WT mice and TLR2- or MyD88-deficient mice as evaluated by flow cytometry. (B) Lineage−/Sca-1+/c-kit+ cells (LSKs), CMPs, promyelocytes, and PMN/band neutrophils were enumerated from infected wounds by flow cytometry. (C) Wounds were collected on day 3 after wounding, and lineage-negative HSPCs were enriched from wound digest using magnetic beads. HSPCs were plated at equal density from all conditions. Ex vivo production of granulocyte-containing colonies from infected vs saline control wounds were counted. Panels A and B represent 5 to 8 mice per group during 2 experiments, and panel C represents 4 mice per group. All data expressed as mean ± SEM for each condition (*P < .05; **P < .01; ***P < .001).
TLR2- or MyD88-deficient mice exhibit diminished granulocyte differentiation in S aureus–infected wounds. Wounds from WT mice, TLR2-deficient, and MyD88-deficient mice were collected 3 days after wounding (+/−S aureus inoculation). (A) Total lineage-negative/c-kit+ HSPCs isolated from wounds of WT mice and TLR2- or MyD88-deficient mice as evaluated by flow cytometry. (B) Lineage−/Sca-1+/c-kit+ cells (LSKs), CMPs, promyelocytes, and PMN/band neutrophils were enumerated from infected wounds by flow cytometry. (C) Wounds were collected on day 3 after wounding, and lineage-negative HSPCs were enriched from wound digest using magnetic beads. HSPCs were plated at equal density from all conditions. Ex vivo production of granulocyte-containing colonies from infected vs saline control wounds were counted. Panels A and B represent 5 to 8 mice per group during 2 experiments, and panel C represents 4 mice per group. All data expressed as mean ± SEM for each condition (*P < .05; **P < .01; ***P < .001).
Next, we examined whether local HSPC differentiation to granulocytes is dependent on signaling via a TLR2/MyD88 pathway. Bead-enriched HSPCs harvested from S aureus–inoculated WT mice were significantly increased compared with the numbers of HSPCs harvested from saline-treated wounds (Figure 3C). Interestingly, HSPCs harvested from both S aureus–inoculated and saline-treated wounds of TLR2- or MyD88-deficient mice formed substantially fewer granulocyte colonies ex vivo than HSPCs harvested from S aureus–inoculated and saline-treated wounds of WT mice (up to a 75% decrease). This mechanism was not the result of an inherent inability of the HSPCs from TLR2- or MyD88-deficient mice to undergo myeloid differentiation, as HSPC numbers in the BM were not different from WT and were competent to form PMN colonies when cultured in methylcellulose media supportive of granulocyte differentiation (supplemental Figures 6A and 7). Thus, granulopoiesis of HSPCs from TLR2- or MyD88-deficient mice is defective within the wound environment but is relatively intact in BM.
TLR2/MyD88 signaling of HSPCs mediates their differentiation within S aureus–infected wounds
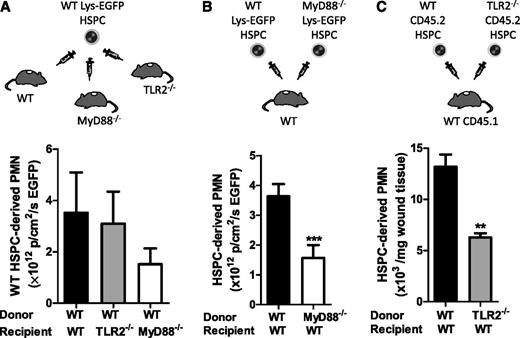
HSPCs differentiate toward myeloid cells on activation by TLR ligands in vitro,5,17 which led to the hypothesis that proliferation and myeloid differentiation at the site of infection was the result of direct recognition of bacterial components by HSPCs, via TLR2 and MyD88, rather than through an indirect mechanism involving cytokines and growth factors present in the wound. BM from WT Lys-EGFP mice was enriched for HSPCs (and included ∼4% LSK cells, or one-third the number of LSKs recruited to S aureus–inoculated wounds) and injected directly into the infected wounds of WT, TLR2-deficient, or MyD88-deficient mice. In these varied wound environments, HSPCs expanded to PMNs equivalently, although there was a trend for less PMN production in MyD88-deficient wounds (Figure 4A). These data indicate that even in the absence of TLR2 or MyD88 signaling in the host cells within infected wounds, WT HSPCs adoptively transferred in these knockouts can undergo normal granulocytic differentiation.
TLR2/MyD88 signaling is required in HSPCs but not in the wound environment for granulopoiesis in S aureus–infected wounds. In panels A and B, infected wounds were injected with HSPCs from transgenic WT or MyD88-deficient Lys-EGFP mice. Fluorescent signal from EGFP-PMNs in the wound was recorded 7 days after injection as a read-out for HSPC differentiation to PMNs. Fluorescence was measured as the total flux of EGFP in photons per centimeters squared per second. (A) BM HSPCs from WT Lys-EGFP mice were directly injected as depicted into S aureus–infected wounds of WT mice and TLR2- or MyD88-deficient mice. EGFP-PMN fluorescence in the wound is shown. (B) BM HSPCs from WT Lys-EGFP mice or MyD88−/−/Lys-EGFP mice were injected into S aureus–infected wounds of WT mice. EGFP-PMN fluorescence in the wound is shown. (C) TLR2-deficient Lys-EGFP mice were not available, so BM HSPCs were harvested from WT or TLR2-deficient mice (CD45.2) and were injected into the S aureus–inoculated wounds of CD45.1 congenic mice. Skin wounds were collected at day 7 after transfer, and the number of donor-derived CD45.2+ PMNs per milligrams of wound tissue was analyzed via flow cytometry. The number of HSPC-produced PMNs is shown as the number of donor-derived CD45.2+ PMNs per milligrams of wound tissue. Data represent 3 mice per group and are displayed as mean ± SEM (**P < .01; ***P < .001).
TLR2/MyD88 signaling is required in HSPCs but not in the wound environment for granulopoiesis in S aureus–infected wounds. In panels A and B, infected wounds were injected with HSPCs from transgenic WT or MyD88-deficient Lys-EGFP mice. Fluorescent signal from EGFP-PMNs in the wound was recorded 7 days after injection as a read-out for HSPC differentiation to PMNs. Fluorescence was measured as the total flux of EGFP in photons per centimeters squared per second. (A) BM HSPCs from WT Lys-EGFP mice were directly injected as depicted into S aureus–infected wounds of WT mice and TLR2- or MyD88-deficient mice. EGFP-PMN fluorescence in the wound is shown. (B) BM HSPCs from WT Lys-EGFP mice or MyD88−/−/Lys-EGFP mice were injected into S aureus–infected wounds of WT mice. EGFP-PMN fluorescence in the wound is shown. (C) TLR2-deficient Lys-EGFP mice were not available, so BM HSPCs were harvested from WT or TLR2-deficient mice (CD45.2) and were injected into the S aureus–inoculated wounds of CD45.1 congenic mice. Skin wounds were collected at day 7 after transfer, and the number of donor-derived CD45.2+ PMNs per milligrams of wound tissue was analyzed via flow cytometry. The number of HSPC-produced PMNs is shown as the number of donor-derived CD45.2+ PMNs per milligrams of wound tissue. Data represent 3 mice per group and are displayed as mean ± SEM (**P < .01; ***P < .001).
Next, we assessed whether HSPCs from TLR2- or MyD88-deficient mice could be induced to differentiate into PMNs when directly transferred to S aureus–inoculated wounds of WT mice. HSPCs from WT Lys-EGFP produced more than twice as many PMNs than did Lys-EGFP/MyD88−/− HSPCs in response to S aureus (P < .001) (Figure 4B). In the absence of Lys-EGFP/TLR2–crossed mice, we performed a similar experiment using HSPCs from CD45.2+ TLR2–deficient mice that were transferred directly into S aureus–inoculated wounds of congenic CD45.1+ WT to quantify their expansion to PMNs. Significantly fewer CD45.2+ TLR2–deficient PMNs were detected compared with HSPCs donated from CD45.2+ WT mice (P < .01) (Figure 4C). To determine whether the differences in PMN production were the result of activation of TLR2 alone, we harvested BM LSK cells from WT and TLR2-deficient mice and incubated them with either no stimulus or Pam3CSK4 in an in vitro expansion assay (Figure 5). Although there was a 50-fold expansion of PMNs from Pam3CSK4–stimulated WT LSK cells, there was no change in PMN production by TLR2-deficient LSK cells. Taken together, these data indicate that HSPCs in the wound differentiate into PMNs via a mechanism that involves direct activation of TLR2/MyD88 signaling.
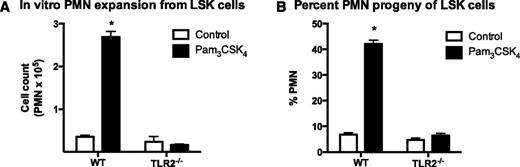
LSK cells isolated from the BM of WT but not TLR2 knockout mice undergo granulopoiesis in response to in vitro Pam3CSK4 stimulation. A total of 5000 FACS LSK cells were plated per well and were stimulated with 1 µg/mL of the TLR2 agonist Pam3CSK4 or vehicle control. Cells were enumerated at day 7. PMN number (A) and percentage (B) for each well was determined by anti-Ly6G staining and flow cytometry. Data represent 3 mice per group and are shown as mean ± SEM (*P < .05).
LSK cells isolated from the BM of WT but not TLR2 knockout mice undergo granulopoiesis in response to in vitro Pam3CSK4 stimulation. A total of 5000 FACS LSK cells were plated per well and were stimulated with 1 µg/mL of the TLR2 agonist Pam3CSK4 or vehicle control. Cells were enumerated at day 7. PMN number (A) and percentage (B) for each well was determined by anti-Ly6G staining and flow cytometry. Data represent 3 mice per group and are shown as mean ± SEM (*P < .05).
S aureus–induced PGE2 production via TLR2/MyD88 supports HSPC proliferation and granulopoiesis within wounds
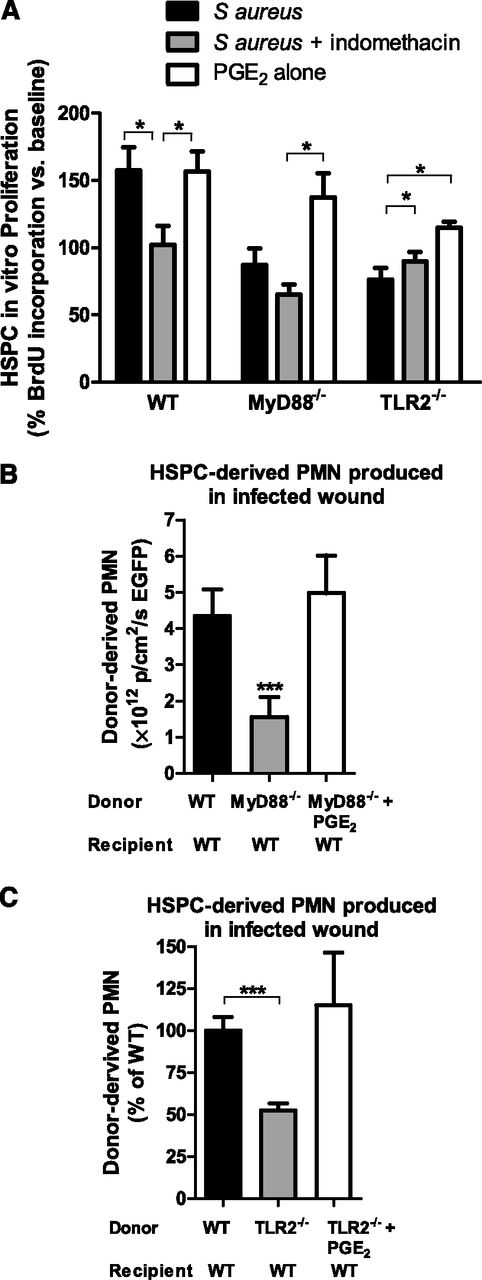
Numerous downstream effector cytokines, chemokines, and growth factors are produced after activation of TLR2/MyD88 signaling that could contribute to HSPC proliferation within inflamed and infected tissue. PGE2 is a key mediator of HSPC survival, proliferation, and homing to BM,9 which led to the hypothesis that PGE2 plays a role in local PMN production from HSPCs within S aureus–inoculated wounds. BM HSPCs from WT mice increased their proliferation by 50% above baseline in vitro in response to heat-killed S aureus as measured by BrdU incorporation (Figure 6A). This increase was abrogated by the addition of indomethacin, which blocks de novo PGE2 production, indicating that enhanced HSPC proliferation in the presence of S aureus was due to PGE2. As expected, S aureus had no effect on proliferation of TLR2- or MyD88-deficient HSPCs. However, the addition of exogenous PGE2 (in the absence of heat-killed S aureus) restored proliferation in TLR2- and MyD88-deficient HSPCs. Together, these data indicate that PGE2 may function as an autocrine proliferation signal in response to S aureus stimulation in vitro.
PGE2 restores proliferation and granulocyte differentiation in HSPCs lacking MyD88 or TLR2. (A) BM HSPCs were incubated for 48 hours in vitro with either heat-killed (HK) S aureus, indomethacin and HK S aureus, or PGE2. BrdU was added for the last 12 hours of incubation to quantify cell proliferation. Data represent 7 to 8 mice per group during 3 experiments and are expressed as a percentage of baseline BrdU incorporation. (B) BM HSPCs from Lys-EGFP WT mice or Lys-EGFPMyD88−/− mice were adoptively transferred into wounds of WT mice with S aureus infection. A third group of mice received HSPCs from Lys-EGFP/MyD88−/− mice that were preincubated with PGE2 before transfer. Total EGFP-PMN signal 7 days after transfer is plotted as a read-out for PMN production. Data represent 3 mice per group. (C) BM HSPCs from CD45.2+ C57BL/6 or TLR2-deficient mice were enriched and transferred to the infected wounds of congenic CD45.1+ mice. A third group received HSPCs from TLR2-deficient mice that were preincubated with PGE2. The number of donor-derived PMNs was determined via flow cytometry and was plotted relative to PMNs produced by donor WT HSPCs. Data represent 4 to 5 mice per group. All data shown as mean ± SEM (*P < .05; ***P < .001).
PGE2 restores proliferation and granulocyte differentiation in HSPCs lacking MyD88 or TLR2. (A) BM HSPCs were incubated for 48 hours in vitro with either heat-killed (HK) S aureus, indomethacin and HK S aureus, or PGE2. BrdU was added for the last 12 hours of incubation to quantify cell proliferation. Data represent 7 to 8 mice per group during 3 experiments and are expressed as a percentage of baseline BrdU incorporation. (B) BM HSPCs from Lys-EGFP WT mice or Lys-EGFPMyD88−/− mice were adoptively transferred into wounds of WT mice with S aureus infection. A third group of mice received HSPCs from Lys-EGFP/MyD88−/− mice that were preincubated with PGE2 before transfer. Total EGFP-PMN signal 7 days after transfer is plotted as a read-out for PMN production. Data represent 3 mice per group. (C) BM HSPCs from CD45.2+ C57BL/6 or TLR2-deficient mice were enriched and transferred to the infected wounds of congenic CD45.1+ mice. A third group received HSPCs from TLR2-deficient mice that were preincubated with PGE2. The number of donor-derived PMNs was determined via flow cytometry and was plotted relative to PMNs produced by donor WT HSPCs. Data represent 4 to 5 mice per group. All data shown as mean ± SEM (*P < .05; ***P < .001).
To determine whether PGE2 could promote HSPC differentiation and PMN expansion within infected wounds, BM HSPCs from WT, MyD88-deficient, or TLR2-deficient mice were preincubated with PGE2 before being transferred into S aureus–inoculated wounds of WT mice (Figure 6B-C). PGE2 was sufficient to restore PMN production in HSPCs from MyD88- or TLR2-deficient donors to levels equivalent to WT. Interestingly, although WT wounds contained large amounts of PGE2 (supplemental Figure 8), this level was not sufficient to recover granulopoiesis of MyD88- or TLR2-deficient HSPCs placed into WT wounds (Figure 4B-C). These results suggest that PGE2 predominantly acted in an autocrine fashion to promote HSPC differentiation. To determine whether PGE2-induced granulopoiesis affected bacterial burden, HSPCs from WT, MyD88-deficient, or TLR2-deficient mice with or without PGE2 treatment were transferred into S aureus–inoculated wounds of MyD88- or TLR2-deficient mice. Pretreatment of HSPCs with PGE2 increased bacterial clearance in the wounds of either MyD88- or TLR2-deficient mice, confirming that PGE2 boosts production of immunocompetent PMNs (supplemental Figure 9A-B).
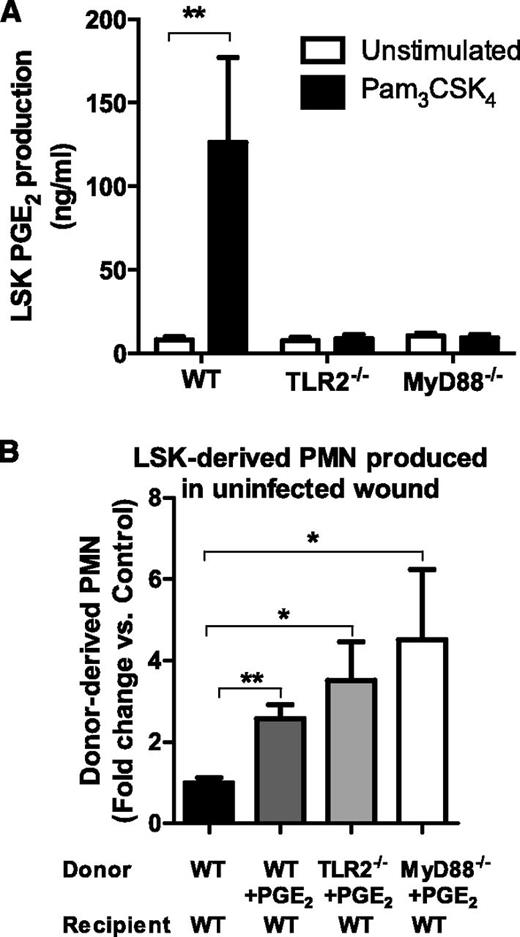
To confirm that TLR2 activation was sufficient to elicit the most proliferative HSPCs to produce PGE2, FACS populations of LSK cells from WT, TLR2-deficient, or MyD88-deficient BM were incubated with or without the TLR2-specific agonist Pam3CSK4. WT LSK cells produced more than 100 ng/mL of PGE2 in response to TLR2 activation, whereas TLR2- or MyD88-deficient LSK produced virtually none (Figure 7A). Finally, to determine whether PGE2 itself was sufficient to promote HSPC differentiation and PMN expansion in uninfected wounds, WT, TLR2-deficient, or MyD88-deficient CD45.2+-LSK cells were incubated with PGE2 and were injected directly into the uninfected wounds of CD45.1+ WT mice. PMN expansion of PGE2-treated LSK cells in WT, TLR2-deficient, or MyD88-deficient mice ranged from 2.5- to 4.5-fold greater than untreated WT LSK cells (Figure 7B). These data reveal that PGE2 is a key downstream product of TLR2/MyD88 signaling and is necessary and sufficient for expansion of LSK cells within cutaneous wounds.
TLR2-activated LSK cells produce PGE2, and PGE2 stimulation of LSK cells results in granulocyte differentiation in the absence of infection. (A) FACS lineage-negative, c-kit, and Sca-1–positive LSK cells were incubated in vitro with the TLR2 agonist Pam3CSK4 or vehicle control, and PGE2 in culture supernatant was measured at 72 hours by enzyme-linked immunosorbent assay. Data represent 3 mice per group during 3 experiments. (B) BM LSK cells derived from CD45.2+-deficient, WT, TLR2-deficient, or MyD88-deficient mice were incubated with PGE2 and were transferred to the uninfected wounds of CD45.1+ mice. The donor-derived CD45.1+-PMN number from the wounds of the respective recipient mice was enumerated by flow cytometry and was plotted relative to the untreated WT LSK cells all in saline-treated wounds 7 days after transfer. Data represent 4 mice per group. All data shown as mean ± SEM (*P < .05; **P < .01).
TLR2-activated LSK cells produce PGE2, and PGE2 stimulation of LSK cells results in granulocyte differentiation in the absence of infection. (A) FACS lineage-negative, c-kit, and Sca-1–positive LSK cells were incubated in vitro with the TLR2 agonist Pam3CSK4 or vehicle control, and PGE2 in culture supernatant was measured at 72 hours by enzyme-linked immunosorbent assay. Data represent 3 mice per group during 3 experiments. (B) BM LSK cells derived from CD45.2+-deficient, WT, TLR2-deficient, or MyD88-deficient mice were incubated with PGE2 and were transferred to the uninfected wounds of CD45.1+ mice. The donor-derived CD45.1+-PMN number from the wounds of the respective recipient mice was enumerated by flow cytometry and was plotted relative to the untreated WT LSK cells all in saline-treated wounds 7 days after transfer. Data represent 4 mice per group. All data shown as mean ± SEM (*P < .05; **P < .01).
Discussion
HSPC cells can enter the circulation and traffic to sites of acute inflammation, where they contribute to the innate immune response.18-21 Our current study reveals a putative mechanism by which HSPCs traffic to cutaneous wounds, sense S aureus, and undergo extramedullary granulopoiesis that is necessary to effectively combat infection. TLR2 detection of pathogen triggers the activity of the adapter protein MyD88 and leads to production of PGE2. This pathway appears to operate in an autocrine manner to signal HSPC expansion and myeloid proliferation during inflammation and infection according to the following lines of evidence: (1) Wounding in the presence of S aureus elicited ∼threefold more HSPC expansion and PMN production in the wound than in the BM where granulopoiesis was amplified more because of inflammation than infection. (2) The presence of TLR2 and MyD88 was necessary for granulopoiesis in the wound in response to S aureus because HSPCs lacking either the receptor or signaling adaptor exhibited defective PMN production, which was rescued by the addition of PGE2. (3) LSK cells produce PGE2 in a TLR2/MyD88–dependent fashion and locally differentiate and can expand PMN numbers solely by stimulation with PGE2. Thus the BM niche is dispensable for local myeloid proliferation, which can be driven locally by PGE2 acting in an autocrine manner to elicit wound granulopoiesis. These findings enhance our understanding of the mechanisms of HSPC function in the periphery at the site of an infection rather than in the BM and point the way for development of targeted therapies to exploit the innate immune function of HSPCs.
We previously demonstrated that S aureus infection of a full-thickness cutaneous wound elicits local HSPC production of PMNs in which the extent of proliferation is proportional to the bacterial burden.3 In our current study, we demonstrate that depletion of HSPCs from the BM by treatment with the anti-c-kit antibody ACK2 effectively diminished PMNs in the abscess by 50%, which was accompanied by a twofold increase in bacterial burden and a ∼30% increase in wound size. By comparing systemic depletion of PMNs by treatment with Gr-1, in the presence or absence of HSPC depletion using ACK2,3 we estimate that ∼30% of PMNs in an abscess arise because of granulopoiesis, and this process is critical to an effective innate immune response. We did not examine mature leukocyte counts in peripheral blood after infection; thus, we do not know the effects of ACK2 antibody on parameters other than PMNs in the wound during infection. It is unlikely that ACK2 exerts its effect through depletion of other leukocyte subsets and their influence on PMN trafficking, because we and other authors have found that ACK2 treatment elicits insignificant reductions in total lymphocyte counts and a mild reduction in hematocrit level.22,23
PMN production in BM is controlled via a feedback mechanism in which homeostasis is regulated by granulocyte colony-stimulating factor (G-CSF), as well as through TLR4 that can trigger its production in response to both neutropenia and during granulopoiesis to meet demand because of infection.24 S aureus signaled through TLR2/MyD88 to elicit a far more robust increase in the relative number of LSKs, CMPs, and promyelocytes in the abscess than in the BM. It is noteworthy that wounding and infection did not cause septicemia, nor did it limit BM myeloid proliferation, which increased ∼sixfold vs baseline and was not significantly increased because of S aureus infection. In contrast, HSPC numbers in the abscess increased by 125-fold vs 16.5-fold in uninfected wounds. Thus, local S aureus infection differentially affects HSPCs in a manner dependent on the local tissue environment. Although saline-treated wounds in our study are likely not sterile, bacterial colony formation detected in saline-treated wound skin samples was very low (<104 colony-forming units [CFUs] at day 5) (Kim and Simon, unpublished data) and likely represent colonization of the wound from the mouse skin commensal microbiota rather than infection with S aureus. Although noninfectious inflammation can elicit recruitment of HSPCs to tissue,4,25 we observed a dose-dependent effect of S aureus inoculation on myeloid progenitor recruitment and differentiation to mature granulocytes in the abscess. As HSPCs are a heterogeneous population, the specific phenotypic target(s) was not identified; however, signaling via TLR2 was demonstrated by in vitro stimulation of LSK cells in a dose-dependent manner by Pam3CSK4. At 1 μg/mL of Pam3CSK4, a 50-fold expansion of PMNs was observed that comprised more than 40% of the total cells in culture at day 7.
Although the numbers of HSPCs in BM of TLR2- and MyD88-deficient mice were equivalent to WT, their numbers were significantly reduced in the wounds of TLR2- or MyD88-deficient mice. It has been shown that infection activates interferon expression, which in turn upregulates the Sca-1 receptor on HSPCs.26 However, cutaneous S aureus infection did not induce significant amounts of interferon-γ. Moreover, it is reported that deficiency in the interferon-γ receptor does not influence wound size or bacterial burden.27 There was no significant difference in the percentage of Sca-1 positivity among lineage-negative cells in S aureus–inoculated wounds vs saline controls (4.1% vs 3.7%, respectively). Indeed, our data indicate that direct recognition of S aureus by TLR2 on HSPCs initiates granulopoiesis in the wound. HSPCs from TLR2- or MyD88-deficient mice produced significantly fewer PMNs compared with WT even when directly transferred into the infected wounds of WT mice to eliminate the variable of trafficking efficiency. TLR2 and MyD88 were required for wound HSPCs to differentiate along the myeloid lineage, as demonstrated by ex vivo colony-forming assays and transfer of HSPCs directly into the infected wounds. Consistent with previous reports,5,17 we observed that stimulation of HSPCs by the TLR2 ligand Pam3CSK4 elicits myelopoiesis, independent of CSFs. The strategy of adoptive transfer of TLR2- and MyD88-deficient HSPCs directly into the wounds of WT mice confirmed that this signaling pathway was necessary and sufficient for local HSPC-dependent myeloid expansion in the wound. Transfer of knockout HSPCs into WT mice with intact immune signaling controlled for potential artifacts caused by a lack of a normal immune response of knockout mice to bacteria. Moreover, bacterial burden was not significantly enhanced in the TLR2 or MyD88 knockouts. These data highlight the primacy of TLR2 activation for elicitation of HSPC differentiation, rather than cytokines produced by cells resident in the wound niche in response to infection and inflammation. Our findings do not rule out an indirect role of other cell types in response to the S aureus infection. Indeed, WT HSPCs exhibited a trend for less efficient granulopoiesis in MyD88-deficient wounds, perhaps because of other upstream receptors including TLRs and/or IL-1R family members. In addition, HSCs upregulate TLR2 in response to G-CSF28 and cutaneous S aureus infection induces γδ T-cell production of IL-17,27 which in turn induces G-CSF production in epithelial cells.29 It is interesting to note that TLR2 signaling enhances myeloid differentiation of blood-derived HSCs far greater than BM-derived HSCs.28 Thus, HSPCs mobilized in response to wounding-induced inflammatory mediators appear primed to respond to TLR2 stimulation and undergo granulopoiesis in the wound.
TLR2 is activated by components of gram-positive bacteria, which leads to upregulation of cytokines, chemokines, and proinflammatory mediators, including PGE2.30 PGE2 modulates levels of tumor necrosis factor alpha,31 a negative regulator of HSPC proliferation,32 as well as IL-6,33 a pleiotropic cytokine that enhances entry into the cell cycle9 and hematopoiesis.34 HSPC homing, survival, and proliferation are augmented after ex vivo incubation with PGE2.9 Moreover, PGE2 acts to expand HSC numbers and increase erythroid and granulocyte/monocyte colonies in a zebrafish model.35 Given the observed effects of PGE2 on HSPC differentiation and its production by LSK cells after TLR stimulation, our data suggest that S aureus–induced PGE2 may serve as an autocrine signal that provides a feed-forward mechanism for enhancement of local proliferation and granulopoiesis. Treatment of LSK cells from TLR2 or MyD88 knockout mice with PGE2 effectively rescued the capacity to produce PMNs even in the absence of bacterial infection in wounds of WT mice. Myeloid progenitors express inducible cyclooxygenase (COX)-1.36 COX inhibition may not only elicit anti-inflammatory effects but may affect the contribution of HSPCs to wound healing. Rather than blocking eicosanoid production, targeting PGE2 receptors may provide a more focused therapeutic effect. PGE2 receptor agonists have been shown to improve healing and prevent ischemic injury in animal models.37,38 Further studies are warranted to determine the benefits and risks of using COX inhibitors or PGE2 receptor agonists in the management of infected wounds.
In summary, these studies delineate an extramedullary immune function for HSPCs at the site of an S aureus cutaneous infection. HSPCs trafficked from the BM to the site of infection, where they were signaled via a pathway involving both TLR2 and MyD88 that promoted expansion from LSK cells and differentiation through CMPs and promyelocytes into mature bactericidal PMNs. This response was linked to the production of PGE2 that acted in an autocrine manner to induce proliferation and granulopoiesis. As HSPCs contribute more than 25% of wound PMNs, targeting of HSPCs via TLR2 or PGE2 receptors may offer a therapeutic advantage in combating antibiotic resistance or augmenting impaired PMN number or function in patients with immunodeficiency. Our report on the physiological role of HSPCs in infection provides insight into how endogenous HSPCs may be manipulated to facilitate resolution of nonhealing and infected wounds.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Thomas Graf generously provided EGFP-lysozyme transgenic mice, and Dr Richard Flavell provided MyD88-deficient mice. Naomi Walker provided excellent technical assistance. The University of California Davis Cancer Center Shared Flow Cytometry Resource and the Center for Molecular and Genomic Imaging provided superb technological support.
This study was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases AI42794 (to S.I.S), AI078910 (to L.S.M.), and T32 AI060555 (to J.L.G.).
Authorship
Contribution: J.L.G., P.C.F., and D.D. performed the experiments; L.S.M. interpreted the data and reviewed the manuscript; J.L.G., D.L.B., and S.I.S. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott I. Simon, Department of Biomedical Engineering, University of California at Davis, 451 E. Health Sciences Dr, Davis, CA 95616; e-mail: sisimon@ucdavis.edu.