In this issue of Blood, Tebas et al report antiviral effects in a clinical trial of multiple infusions of lentiviral vector–modified autologous CD4 T lymphocytes in 17 HIV-infected patients aviremic on antiretroviral therapy (ART).1
The conditionally replicating lentiviral vector (VRX496) expressed an anti-sense gene complementary to HIV envelope. Twelve participants received 6 infusions of the modified cells; the remaining 5 received 3 infusions after it was observed that additional infusions did not improve persistence of the vector-modified cells. Thirteen patients underwent an analytical antiretroviral treatment interruption (ATI) to determine the effect of the intervention on the new plasma HIV RNA set point. Of these 13 patients, 6 of the 8 with historic pre-ART values had lower values at the end of the ATI. This potential antiviral effect, although modest and not quite achieving statistical significance, was supported by the finding that the vector-modified cells exerted genetic pressure on the HIV present in the patients, with enriched A-to-G transitions in the anti-sense region of the HIV envelope. The antiviral activity needs to be confirmed in prospective, randomized, placebo-controlled clinical trials.
The administration of the modified T cells appeared to be safe, with no unexpected adverse effects related to the study treatment. Fever, chills, and other reactions to the cytokines released by infused T cells can be alleviated by pre-infusion anti-pyretics and antihistamines. No evidence of integration enrichment near known oncogenes was found. Conditional replication of the lentivirus vector was detected, presumably as a result of induction by HIV infection of transduced cells.
As in other clinical studies of the reinfusion of autologous CD4 T cells,2 the CD4 T-cell count rose in the study patients. This was probably largely due to the current use of CD28 in addition to CD3 to stimulate and propagate the CD4 T cells ex vivo before reinfusion, and not necessarily due to the specific gene insertion. The addition of CD28 was a major advance that facilitated the large-scale expansion of CD4 T cells for clinical use.2 It is likely that there was an initial expansion of the infused activated T cells as well as a generalized peripheral expansion of lymphocytes induced by cytokines released from the infused T cells. Infused activated T cells are known to express Ki-67, a marker of cell proliferation, and the percentage of CD4+Ki-67+ cells increases in patients receiving these infusions beyond what would be expected from the contribution of the infused cells.2 The percentage of cells induced to produce IL-2, a cytokine that promotes cellular proliferation, also increases.2 Initial experiments suggest that these infused cells are capable of proliferating to alloantigens,2 but additional functional analyses should be performed. Ultimately, the clinical significance of the increased CD4 T-cell counts would need to be established through clinical event end point trials. The IL-2 experience chastened us in this regard.3
The infused cells in the Tebas study persisted in patients for up to 5 years, but the half-life in blood was only 5 weeks.1 The durability of the modified T cells seemed to plateau after 3 infusions. Importantly, engraftment of cells in the gut lymphoid tissue was comparable with that in blood. The basis for the limited half-life of the modified cells compared with other gene therapy approaches is unclear, as humoral or cellular immune responses against the product were not induced. Prolonging the persistence of these gene-modified blood cells and other gene therapy products may require cytoreductive “conditioning” to reduce competition from unmodified cells for trophic factors.1 Unfortunately, this would increase the risk of toxicity for patients who otherwise have access to extremely effective suppressive chemotherapy.
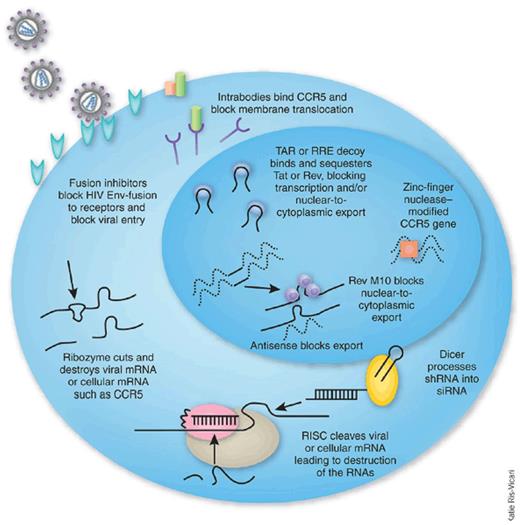
As with antiretroviral chemotherapy, gene therapy approaches to inhibiting HIV infection are directed at different steps in the life cycle of the virus (see figure).4 Transgenes code for either an RNA-based agent, as in this trial, or a protein-based agent. The agent may target the virus or a host cell element needed in the life cycle of the virus. For the latter, the candidate treatment should avoid disruption of essential cell function, and clinical trials need to monitor for this accordingly.
Inhibitory agents used in HIV hematopoietic cell gene therapy trials. Reprinted from Rossi et al4 with permission.
Inhibitory agents used in HIV hematopoietic cell gene therapy trials. Reprinted from Rossi et al4 with permission.
Hematopoietic stem cells (HSCs) have advantages over CD4 T lymphocytes as cell targets for HIV gene therapy.4 They account for all the lineages of cells subject to infection by HIV. Transduced HSCs persist longer than transduced CD4 T cells. On the other hand, HSCs do not expand readily ex vivo, thus having a greater need for cytoreductive conditioning in patients for better engraftment. They take longer to proliferate in vivo after infusion, but proliferate widely once they start. The latter property results in a greater risk of insertional oncogenesis.
To enhance cytotoxic T-lymphocyte activity of CD8 T cells, genes can be inserted into CD8 T lymphocytes to create a CD4 extracellular domain, or other HIV envelope–binding molecule or antibody, coupled to the ζ signaling chain of the CD3 T-cell receptor (TCR).5 Another strategy is to redirect the TCRs of the CD8 T cells to target specific HIV antigens. The latter approach has been successful in the treatment of chronic lymphoid leukemia.6 A risk is potential off-target immunogenicity when modified TCR chains pair with the native chains.
The antiretroviral effects of the VRX496-modified CD4 T cells were modest. Other gene therapy approaches have demonstrated modest antiviral activity.5,7,8 It is to be expected that, as with antiretroviral chemotherapy, each treatment approach individually will be incomplete in its ability to fully contain viral replication and that the development of viral resistance will become an important limitation when used alone. Thus, combinations of gene therapy agents may be needed to obtain optimal control of the infection.
Current antiretroviral therapy is merely suppressive, as HIV establishes latency in the genome of resting memory CD4 T lymphocytes.9 The development of gene therapy for HIV infection holds the promise of creating a body of cells that can resist HIV and permit a longer lasting control of the infection perhaps without the need for antiretroviral drugs.
Conflict-of-interest disclosure: The author served on a data and safety monitoring board for an HIV gene therapy study sponsored by Sangamo Biosciences. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal