To the editor:
In a recent issue of Blood, Ran et al1 reported that ectopic expression of RUNX1a isoform facilitates the emergence of definitive hematopoietic stem/progenitor cells (HSPCs) from human embryonic stem cells (hESCs), as well as an impressive expansion potential of the RUNX1a-hESC–derived HSPCs, eventually conferring multilineage in vivo engraftment ability.
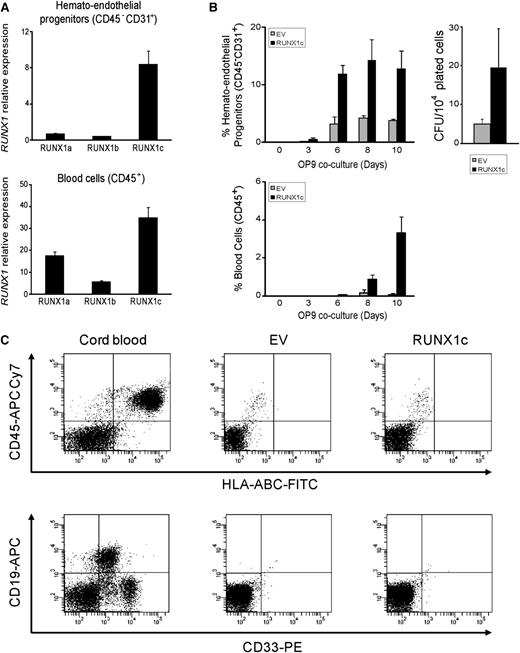
The function of RUNX1 isoforms in human blood specification remains elusive and controversial.2 Ran et al1 showed in bulk hESC-differentiating cultures that RUNX1b/c isoforms are higher expressed than RUNX1a very early in specification (before blood emergence), whereas RUNX1b/c expression is lower than RUNX1a at the time of or after blood emergence. However, our data using different hESC lines (H9, AND1, HS181) and both embryoid bodies–based and OP9 coculture–based differentiation systems3 reveal that the expression of RUNX1c emerges significantly before RUNX1a and RUNX1b (Figure 1A). RUNX1c is almost the only RUNX1 isoform expressed in hESC-derived fluorescence-activated cell sorting (FACS)-purified hemato-endothelial progenitors (CD31+CD45–) and the highest expressed in FACS-purified hESCs-derived CD45+ blood cells (Figure 1A). Furthermore, lentiviral-mediated expression of RUNX1c robustly accelerates and enhances the appearance of hemato-endothelial progenitors, hematopoietic cells, and colony-forming unit potential (Figure 1B). However, despite robust hematopoietic enhancement in vitro, RUNX1c does not confer engraftment potential in newborn immunodeficient SCID–IL2Rgamma(null) mice, in keeping with previous work in mouse HSPCs4 (Figure 1C).
RUNX1c isoform accelerates and enhances hematopoietic commitment of hESC. (A) Quantitative reverse-transcriptase polymerase chain reaction analysis of the 3 main RUNX1 isoforms in the H9 hESC cell line shows that RUNX1c is the isoform almost exclusively expressed in CD31+CD45– hemato-endothelial progenitors, and is expressed at higher levels in CD45+ hematopoietic cells. Relative expression is shown normalized to undifferentiated hESCs. (B) Kinetics of hematopoietic specification from H9 hESCs transduced with the empty vector (EV) or RUNX1c-expressing vector (RUNX1c). RUNX1c accelerates and enhances hematopoietic development of hESCs. (C) Between 1 × 105 and 2.5 × 105 hESC-derived EV or RUNX1c hematopoietic derivatives were transplanted intrahepatically into newborn immunodeficient SCID–IL2Rgamma(null) mice.14 RUNX1c-hESC hematopoietic derivatives fail to engraft in vivo. FACS analysis was performed 7 to 8 weeks after transplantation (n = 12). Cord blood–CD34+ cells were transplanted as a positive control. APC, allophycocyanin; CFU, colony-forming units; FITC, fluorescein isothiocyanate; PE, Phycoerythrin.
RUNX1c isoform accelerates and enhances hematopoietic commitment of hESC. (A) Quantitative reverse-transcriptase polymerase chain reaction analysis of the 3 main RUNX1 isoforms in the H9 hESC cell line shows that RUNX1c is the isoform almost exclusively expressed in CD31+CD45– hemato-endothelial progenitors, and is expressed at higher levels in CD45+ hematopoietic cells. Relative expression is shown normalized to undifferentiated hESCs. (B) Kinetics of hematopoietic specification from H9 hESCs transduced with the empty vector (EV) or RUNX1c-expressing vector (RUNX1c). RUNX1c accelerates and enhances hematopoietic development of hESCs. (C) Between 1 × 105 and 2.5 × 105 hESC-derived EV or RUNX1c hematopoietic derivatives were transplanted intrahepatically into newborn immunodeficient SCID–IL2Rgamma(null) mice.14 RUNX1c-hESC hematopoietic derivatives fail to engraft in vivo. FACS analysis was performed 7 to 8 weeks after transplantation (n = 12). Cord blood–CD34+ cells were transplanted as a positive control. APC, allophycocyanin; CFU, colony-forming units; FITC, fluorescein isothiocyanate; PE, Phycoerythrin.
RUNX1 is a master hematopoietic transcription factor that acts as an oncogene in several childhood leukemias,5,6 mixed-lineage–rearranged leukemias,7 and T-cell lymphoma.8 In addition, this laboratory has previously shown that overexpression of RUNX1a strongly contributes to leukemogenesis.9 This occurs because RUNX1a is missing the transactivation domain essential for its normal function in hematopoiesis,9,10 and consequently it functions as a dominant inhibitor of other RUNX1 isoforms.9,10
This “double-edged sword” of RUNX111 argues whether the data reported by Ran et al1 are a consequence of RUNX1a-mediated transformation of hESC-derived blood cells.12 Our concern is based on the following data reported by Ran et al: (i) RUNX1a is massively (>700-fold) and nonphysiologically overexpressed; (ii) RUNX1a-hESC–derived CD45+CD34+ HSPCs surprisingly expand 25-fold more than the control within a short (7-day) window; (iii) RUNX1a-hESC–derived CD45+CD34+ HSPCs expand even more than their somatic CB-CD34+ counterparts in stroma cocultures; (iv) ∼80% of the CD45+ hematopoietic stem cells (HSCs) remain as CD45+CD34+, suggestive of a lack of terminal differentiation into CD45+CD34– cells; and (v) 100% of the mice analyzed displayed engraftment (CD45+), which, to date, represents a major stumbling block in the field. However, when the multilineage engraftment was analyzed, the proportion of myeloid cells (CD33+), B-lymphoid cells (CD19+), and erythroid cells (CD36+) add up to as little as 23% of the graft. The question then is: What is the phenotype of the remaining 77% of the cells within the graft? Because these data are not shown, we propose that they may be CD45+CD34+ cells displaying a differentiation blockage.
Finally, the large expansion of the RUNX1a-hESC–derived CD45+CD34+ HSPCs allowed the authors to transplant as many as 150 000 CD45+CD34+ cells, which is typically not possible for laboratories worldwide. Because in vivo data on wild-type or empty vector–expressing hESCs is not shown, it is difficult to conclude whether the engraftment is directly attributed to RUNX1a-mediated self-renewal/specification effects or whether it may be an indirect consequence of the large number of CD45+CD34+ cells that were transplanted. To resolve this issue, RUNX1a knockdown experiments would be helpful to show that the phenotype is causatively related to RUNX1a expression.
We conclude that the RUNX1c isoform appears to parallel hematopoietic emergence better than RUNX1a, and that overexpression of either RUNX1a or RUNX1c enhances the hemato-endothelial and hematopoietic potential of hESCs in vitro. However, the hematopoietic derivatives from RUNX1a-hESCs and RUNX1c-hESCs seem to display differential in vivo engraftment ability, as previously described in transplantation experiments using mouse HSCs.4,13 In addition, the surrogate assays conducted to assess in vitro and in vivo the hematopoietic potential of hESC hematopoietic derivatives should be interpreted with caution to thoroughly address the direct versus indirect consequences of ectopically overexpressed transcription factors involved in leukemogenesis. Nevertheless, regardless of the fact that the data from Ran et al1 could be the result of either normal hematopoietic specification or a leukemogenic effect, the data are equally relevant for the field. This is because many efforts are being undertaken in both directions: (i) generation of in vivo functional HSCs from hESCs and (ii) understanding of the molecular/developmental mechanisms underlying newborn/childhood leukemias with a very well-established embryonic/fetal origin.
Authorship
Acknowledgments: This work is supported by grants from The Instituto de Salud Carlos III-FEDER (PI10/0449 [P.M.], CP09/0063 and PI12/01598 [P.J.R.], CP07/0059 and PI11/00119 [C.B.], and CP12/03175 [V.R-M.]); The Spanish Association Against Cancer (P.M.); Health Canada (H4084-112281 [P.M.]); and the CSJA (0029/2006 and 0030/2006 [P.M.]; SAS-111244 [P.J.R.]) and CICE (P08-CTS-3678 [P.M.] and P10-CTS-6406 [P.J.R.]) de la Junta de Andalucía.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pedro J. Real and Pablo Menendez, Avda de la Ilustración 114-PTS, Granada 18016, Spain; e-mail: pedro.real@genyo.es, pmenendez@carrerasresearch.org.
References
Author notes
P.J.R. and O.N.-M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal