In this issue of Blood, Ruan et al demonstrate that the tyrosine kinase inhibitor (TKI), imatinib, can cause regression of human lymphomas in vivo; interestingly, this occurs via antiangiogenesis mediated by disruption of the platelet-derived growth factor receptor-β positive (PDGFRβ+) pericyte.1
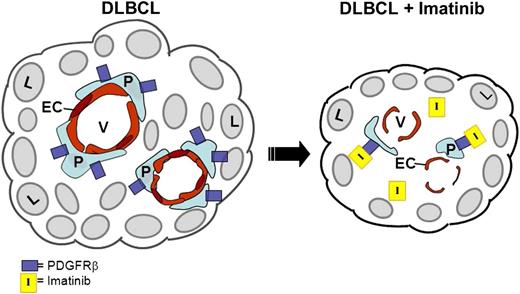
Schematic representation of antiangiogenic effects of imatinib on DLBCL. On the left, intact lymphoma vasculature is represented. On the right, in the presence of imatinib, PDGFRβ+ pericytes (P) are depleted via imatinib binding to the PDGFRβ receptor, leading to EC apoptosis, disruption of tumor vessel (V) integrity, and lymphoma (L) regression.
Schematic representation of antiangiogenic effects of imatinib on DLBCL. On the left, intact lymphoma vasculature is represented. On the right, in the presence of imatinib, PDGFRβ+ pericytes (P) are depleted via imatinib binding to the PDGFRβ receptor, leading to EC apoptosis, disruption of tumor vessel (V) integrity, and lymphoma (L) regression.
Investigators have long postulated that the tumor microenvironment, and particularly the microvasculature, modulates the pathogenesis of diffuse large cell lymphoma.2 More recently, genomic analyses of human diffuse large B-cell lymphomas (DLBCLs) have suggested that signatures of increased tumor vascular density correlated strongly with adverse prognosis.3 However, clinical trials using an anti–vascular endothelial growth factor (VEGF) antibody (bevacizumab) in an attempt to inhibit lymphoma angiogenesis in patients with DLBCL or mantle cell lymphoma failed to show meaningful antitumor efficacy.4,5 In this issue of Blood, Ruan et al1 describe a unique strategy to achieve antiangiogenesis and lymphoma regression via targeting of the PDGFRβ+ pericyte or vascular smooth muscle cell, which is responsible for structural and paracrine-mediated support of vascular endothelium.6,7 Using either imatinib, a TKI that targets PDGFRβ among other kinases, or a PDGFRβ-specific antibody, the authors show regression and apoptosis of pericytes and inhibition of lymphoma growth in mouse models. The authors compared the growth of 3 different human DLBCL lines in SCID mice and found that 2 to 3 weeks of imatinib treatment caused significant shrinkage of all 3 tumors, coupled with intratumoral regression and apoptosis of PDGFRβ+ pericytes. Depletion of PDGFRβ+ pericytes was also associated with increased apoptosis of CD31+ vascular endothelial cells (ECs) and gross disruption of the tumor vasculature in vivo (see figure).
The findings presented here raise several interesting questions: First, because TKIs can have off-target effects and affect several cell types, did imatinib work specifically via disruption of PDGFRβ+ pericytes or were the lymphoma cells or other microenvironment cell types affected as well? Interestingly, the authors show that none of the human lymphoma cells expressed PDGFRβ and <1% of the vascular ECs expressed PDGFRβ, suggesting that PDGFRβ+ pericytes were the specific target of imatinib in this model. Commensurate with this, imatinib imparted strong growth inhibition against PDGFRβ+ vascular smooth muscle cells in vitro, whereas human lymphoma cells and ECs displayed high and intermediated resistance, respectively, to imatinib in vitro. Importantly, siRNA-mediated silencing of PDGFRβ inhibited the growth of human pericytes in vitro and rendered these cells insensitive to imatinib-mediated toxicity. Conversely, knockdown of PDGFRβ in human lymphoma cells had no effect on either cell growth or resistance to imatinib compared with controls. Taken together, these data suggested that imatinib acted specifically via antagonism of PDGFRβ signaling in pericytes in mediating the antilymphoma effects.
Second, how does imatinib-mediated disruption of pericytes cause such profound vascular disruption and lymphoma regression? In this study, imatinib-mediated inhibition of PDGFRβ signaling in vascular smooth muscle cells caused a significant reduction in activation of PDGFRβ, PDGFRα, and c-kit, as well as the downstream effectors Akt and mitogen-activated protein kinase. Furthermore, imatinib treatment blunted vascular smooth muscle cell expression and secretion of VEGF and transforming growth factor β, which are both important in maintaining the survival, proliferation, and integrity of vascular ECs.6,7 The latter findings provide the basis for understanding mechanistically how imatinib-mediated disruption of PDGFRβ+ pericytes can cause such profound antiangiogenic effects in the absence of direct targeting of endothelium.
It is worth noting in this study that treatment of mice with 2C5, an anti-PDGFRβ–specific antibody, caused substantial depletion of NG2+ pericytes in human lymphomas in vivo but more modest effects on tumor vascular density and tumor growth in vivo compared with imatinib. This may be explained by the persistence of perivascular CD68+ myeloid cells in anti-PDGFRβ–treated lymphomas, which were otherwise depleted in response to imatinib. The authors postulate that CD68+ perivascular myeloid cells may serve as surrogates to maintain vascular integrity in tumors in the face of targeted depletion of pericytes. As pointed out by the authors, the stronger antilymphoma effects of imatinib compared with the anti-PDGFRβ antibody may reflect the activity of imatinib against a wider range of stromal cell targets, including PDGFRα+ fibroblasts and c-fms+ myelomonocytic cells, as well as via inhibition of c-kit signaling in pericytes.
Taken together, this study provides proof of principle that administration of a TKI can mediate antiangiogenesis in a human lymphoma model via inhibition of PDGFRβ+ pericyte function. Importantly, this approach resulted in significant inhibition of human lymphoma growth in vivo. These interesting results provide the basis for more refined consideration of antiangiogenesis as a strategy to attack human lymphomas in the clinic. In that regard, it is also encouraging that the authors observed a differential toxicity of imatinib therapy against the lymphoma vasculature, whereas the vasculatures of organs not involved with lymphoma were not affected. It has been suggested that one reason anti-VEGF therapy has not succeeded in the treatment of patients with lymphoma is that other proangiogenic mechanisms were not disabled via this approach.4 Targeting the PDGFRβ+ pericyte, which regulates multiple pathways involved in the maintenance and proliferation of vascular ECs, represents an attractive strategy to dismantle the lymphoma vasculature and, perhaps, the lymphoma itself.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal