Key Points
Incidence of sclerotic GVHD is 20% by 3 years after initial systemic treatment for chronic GVHD.
The use of mobilized blood cell graft and total body irradiation conditioning are associated with an increased risk of sclerotic GVHD.
Abstract
Sclerotic chronic graft-versus-host disease (GVHD) can result in disability after allogeneic hematopoietic cell transplantation. We assessed the incidence and risk factors of sclerosis and its association with transplant outcomes among 977 consecutive patients treated with systemic immunosuppression for chronic GVHD. Sclerosis was defined when cutaneous sclerosis, fasciitis, or joint contracture was first documented in the medical record. Seventy (7%) patients presented with sclerosis at the time of initial systemic treatment for chronic GVHD, and the cumulative incidence of sclerosis increased to 20% at 3 years. Factors associated with an increased risk of sclerosis included the use of a mobilized blood cell graft and a conditioning regimen with >450 cGy total body irradiation. Factors associated with a decreased risk of sclerosis included the use of an HLA-mismatched donor and a major ABO-mismatched donor. Development of sclerosis was associated with longer time to withdrawal of immunosuppressive treatment but not with risks of overall mortality, nonrelapse mortality, or recurrent malignancy. We found a substantial incidence of sclerosis in patients with chronic GVHD. Development of sclerosis can cause disability but does not affect mortality or recurrent malignancy in patients with chronic GVHD.
Introduction
Sclerotic graft-versus-host disease (GVHD) represents a distinctive phenotype of chronic GVHD first described in 19771 ; it is often associated with severe disability and morbidity after allogeneic hematopoietic cell transplantation (HCT).2 While sclerotic GVHD has some clinical and histopathological similarities with systemic sclerosis, the pathogenic mechanisms of the 2 diseases are thought to differ.3,4 Systemic sclerosis can involve visceral organs with pulmonary hypertension, renal dysfunction, and cardiac dysfunction, whereas these manifestations are rarely observed in patients with sclerotic GVHD.2,3 Systemic sclerosis begins in deeper layers of the skin and then extends to superficial layers, whereas sclerotic GVHD usually begins in superficial layers of the skin and then extends to deeper layers.2,4 Systemic sclerosis often presents with vasculopathy and Raynaud’s phenomenon,3 whereas sclerotic GVHD rarely presents with these manifestations,2,4 although endothelial injury associated with chronic GVHD has been observed in some studies.5
To date, reports describing sclerosis among patients with chronic GVHD have been limited to small series and 1 larger cross-sectional study of single-patient visits for an evaluation of relatively severe chronic GVHD.6-8 The incidence of sclerotic GVHD, risk factors for sclerotic GVHD, and its association with major transplant outcomes have not been well defined. While chronic GVHD occurs in 40% to 50% of patients after HCT, some patients develop sclerosis during the clinical course of chronic GVHD, but others do not. In a large cohort of consecutive patients treated with systemic immunosuppression for chronic GVHD, we aimed to elucidate the following: (1) the overall incidence of sclerosis, (2) risk factors for sclerosis, and (3) potential differences in transplant outcomes after patients develop sclerosis. An increased understanding of risk factors would help to identify candidates for early interventional studies aimed at reducing disability related to sclerosis after HCT.
Methods
Patients
We identified all patients who received initial systemic treatment for chronic GVHD after a first allogeneic HCT between May 2000 and December 2009. Treatment of malignant or nonmalignant diseases was provided at the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance, Seattle, WA. Patients who received a double cord blood transplantation were excluded because the interpretation of donor-related factors such as gender combination, HLA matching, and ABO matching in this setting is complex.9 Patients who had recurrent malignancy before the onset of chronic GVHD were excluded because immunosuppressive treatment might have been modified in order to enhance graft-versus-tumor effect after recurrent malignancy. Patients gave written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki, and the Fred Hutchinson Cancer Research Center institutional review board approved the study.
Clinical assessments and definitions
Acute GVHD was graded according to previously described criteria.10 Chronic GVHD was diagnosed using National Institutes of Health (NIH) consensus criteria.11 Information about chronic GVHD and follow-up outcomes were captured prospectively via the Long-Term Follow-Up Program through medical records from our outpatient clinic and local clinics that provided primary care for patients.12 Sclerotic GVHD was defined when manifestations of cutaneous sclerosis, fasciitis, or joint contracture were first documented in the medical record. A comprehensive retrospective chart review was performed to identify an accurate onset date and manifestations of sclerotic GVHD. HLA typing of donor and recipient pairs was performed using methods informative for the highest resolution of alleles available at the time of the analysis. Recipient HLA mismatching was defined when a recipient had at least 1 HLA-A, B, C, DRB1, or DQB1 antigen or allele that was not present in the donor (ie, GVHD vector mismatching).
Statistical analysis
The primary endpoint was development of sclerosis, as defined above, at any time among all patients with chronic GVHD included in the study. The cumulative incidence of sclerosis was estimated based on the initial systemic treatment for chronic GVHD, treatment for recurrent malignancy, and death without sclerosis as competing risks.13 Cox regression analyses were used to identify risk factors for sclerosis. The first analysis examined patient and transplant-related risk factors for sclerosis at any time among all patients with chronic GVHD. In this analysis, sclerosis present at the onset of systemic treatment for chronic GVHD was treated as an event at day 1 after treatment. The second analysis examined chronic GVHD-related risk factors for subsequent development of sclerosis among patients who did not have sclerosis at the onset of systemic treatment for chronic GVHD. Patient and transplant-related variables considered in Cox regression analyses included patient and donor age per decade, patient gender, donor–recipient gender combination, disease risk, stem cell graft source, donor relation, HLA matching, ABO compatibility, intensity of conditioning regimen, total body irradiation (TBI) dose, use of rabbit antithymocyte globulin in the conditioning regimen, GVHD prophylaxis regimen, prior grade II to IV acute GVHD, and prior history of severe skin acute GVHD (stage 3 or 4). Chronic GVHD-related variables evaluated at the onset of initial systemic treatment included presence of eosinophilia (>400/μL), bronchiolitis obliterans and thrombocytopenia (<100 000/μL), and progressive onset (ie, direct progression from acute GVHD to chronic GVHD or onset of chronic GVHD during steroid treatment). Factors with a P value ≤ .05 for association with sclerosis in univariate testing were entered in a multivariate Cox regression model. A backward elimination procedure was used to exclude risk factors until the P value of the likelihood ratio test for all remaining risk factors was ≤.05. A similar procedure was used to evaluate risk factors for mortality.
As secondary endpoints, overall mortality, nonrelapse mortality (malignant disease only), recurrent malignancy (malignant disease only), and end of systemic immunosuppressive treatment were compared between chronic GVHD patients with and without sclerosis, treating all sclerosis at any time as a time-dependent risk factor. Hazard ratios (HRs) were adjusted for covariates associated with overall mortality in previous studies.12,14 The analysis was carried out in July 2012.
Results
Patient characteristics and incidence of sclerosis in patients with chronic GVHD
We identified 1064 consecutive patients who received initial systemic treatment for chronic GVHD after a first allogeneic HCT between May 2000 and December 2009. A total of 87 patients were excluded because they had double cord blood transplantation (n = 18) or recurrent malignancy before chronic GVHD (n = 69). The final study cohort included 977 patients with chronic GVHD. The median age of the patients was 48 years (range, 0–78 years). The median time from HCT to initial systemic treatment for chronic GVHD was 5.3 months (range, 2.5–33.5 months). The median follow-up after chronic GVHD among survivors was 80 months (range, 6–135 months). Other demographics of the study cohort are summarized in Table 1.
Characteristics of the study cohort
| Characteristic . | Cohort, N = 977 . |
|---|---|
| Median time from transplantation to initial systemic treatment for chronic GVHD, mo (range) | 5.3 (2.5–33.5) |
| Median patient age at transplantation, y (range) | 48 (0–78) |
| Median donor age at transplantation, y (range) | 39 (0–78) |
| Patient gender, no. (%) | |
| Male | 565 (58) |
| Female | 412 (42) |
| Donor–recipient gender combination, no. (%) | |
| Male to male | 297 (30) |
| Female to male | 268 (27) |
| Male to female | 179 (18) |
| Female to female | 233 (24) |
| Patient race, no. (%) | |
| Caucasian | 766 (78) |
| African American | 16 (2) |
| Other | 166 (17) |
| Missing data | 29 (3) |
| Diagnosis, no. (%) | |
| Acute myeloid leukemia | 301 (31) |
| Acute lymphoid leukemia | 105 (11) |
| Chronic myeloid leukemia | 108 (11) |
| Myelodysplastic syndromes or myeloproliferative neoplasms | 212 (22) |
| Chronic lymphocytic leukemia | 40 (4) |
| Malignant lymphoma | 105 (11) |
| Multiple myeloma | 59 (6) |
| Aplastic anemia | 14 (1) |
| Other | 33 (3) |
| Disease risk,* no. (%) | |
| Low | 337 (34) |
| High | 640 (66) |
| Stem cell graft source, no. (%) | |
| Bone marrow | 143 (15) |
| Mobilized blood cells | 820 (84) |
| Umbilical cord blood | 14 (1) |
| HLA and donor type, no. (%) | |
| HLA matched related | 406 (42) |
| HLA matched unrelated | 373 (38) |
| HLA mismatched related | 36 (4) |
| HLA mismatched unrelated | 162 (17) |
| ABO compatibility, no. (%) | |
| Match | 533 (55) |
| Minor mismatch | 197 (20) |
| Major mismatch | 247 (25) |
| Intensity of conditioning regimen, no. (%) | |
| High | 693 (71) |
| Reduced | 284 (29) |
| TBI dose in conditioning regimen, no. (%) | |
| None | 385 (39) |
| ≤450 cGy | 360 (37) |
| >450 cGy | 232 (24) |
| ATG in conditioning regimen, no. (%) | 54 (6) |
| GVHD prophylaxis, no. (%) | |
| Cyclosporine + MTX/MMF | 593 (61) |
| Tacrolimus + MTX/MMF | 350 (36) |
| Other | 34 (3) |
| Prior grade II–IV acute GVHD, no. (%) | 725 (74) |
| Prior stage 3–4 skin acute GVHD, no. (%) | 359 (37) |
| Sites involved with chronic GVHD at initial systemic treatment, no. (%) | |
| Skin | 674 (69) |
| Eye | 267 (27) |
| Mouth | 740 (76) |
| Gastrointestinal tract | 374 (38) |
| Liver | 254 (26) |
| Lung (bronchiolitis obliterans) | 16 (2) |
| Joint or fascia | 63 (6) |
| Genital tract | 45 (5) |
| Eosinophilia >400/μL at initial systemic treatment, no. (%) | 156 (16) |
| Thrombocytopenia <100 000/μL at initial systemic treatment, no. (%) | 318 (33) |
| Progressive onset,† no. (%) | 339 (35) |
| Characteristic . | Cohort, N = 977 . |
|---|---|
| Median time from transplantation to initial systemic treatment for chronic GVHD, mo (range) | 5.3 (2.5–33.5) |
| Median patient age at transplantation, y (range) | 48 (0–78) |
| Median donor age at transplantation, y (range) | 39 (0–78) |
| Patient gender, no. (%) | |
| Male | 565 (58) |
| Female | 412 (42) |
| Donor–recipient gender combination, no. (%) | |
| Male to male | 297 (30) |
| Female to male | 268 (27) |
| Male to female | 179 (18) |
| Female to female | 233 (24) |
| Patient race, no. (%) | |
| Caucasian | 766 (78) |
| African American | 16 (2) |
| Other | 166 (17) |
| Missing data | 29 (3) |
| Diagnosis, no. (%) | |
| Acute myeloid leukemia | 301 (31) |
| Acute lymphoid leukemia | 105 (11) |
| Chronic myeloid leukemia | 108 (11) |
| Myelodysplastic syndromes or myeloproliferative neoplasms | 212 (22) |
| Chronic lymphocytic leukemia | 40 (4) |
| Malignant lymphoma | 105 (11) |
| Multiple myeloma | 59 (6) |
| Aplastic anemia | 14 (1) |
| Other | 33 (3) |
| Disease risk,* no. (%) | |
| Low | 337 (34) |
| High | 640 (66) |
| Stem cell graft source, no. (%) | |
| Bone marrow | 143 (15) |
| Mobilized blood cells | 820 (84) |
| Umbilical cord blood | 14 (1) |
| HLA and donor type, no. (%) | |
| HLA matched related | 406 (42) |
| HLA matched unrelated | 373 (38) |
| HLA mismatched related | 36 (4) |
| HLA mismatched unrelated | 162 (17) |
| ABO compatibility, no. (%) | |
| Match | 533 (55) |
| Minor mismatch | 197 (20) |
| Major mismatch | 247 (25) |
| Intensity of conditioning regimen, no. (%) | |
| High | 693 (71) |
| Reduced | 284 (29) |
| TBI dose in conditioning regimen, no. (%) | |
| None | 385 (39) |
| ≤450 cGy | 360 (37) |
| >450 cGy | 232 (24) |
| ATG in conditioning regimen, no. (%) | 54 (6) |
| GVHD prophylaxis, no. (%) | |
| Cyclosporine + MTX/MMF | 593 (61) |
| Tacrolimus + MTX/MMF | 350 (36) |
| Other | 34 (3) |
| Prior grade II–IV acute GVHD, no. (%) | 725 (74) |
| Prior stage 3–4 skin acute GVHD, no. (%) | 359 (37) |
| Sites involved with chronic GVHD at initial systemic treatment, no. (%) | |
| Skin | 674 (69) |
| Eye | 267 (27) |
| Mouth | 740 (76) |
| Gastrointestinal tract | 374 (38) |
| Liver | 254 (26) |
| Lung (bronchiolitis obliterans) | 16 (2) |
| Joint or fascia | 63 (6) |
| Genital tract | 45 (5) |
| Eosinophilia >400/μL at initial systemic treatment, no. (%) | 156 (16) |
| Thrombocytopenia <100 000/μL at initial systemic treatment, no. (%) | 318 (33) |
| Progressive onset,† no. (%) | 339 (35) |
ATG, antithymocyte globulin; MTX, methotrexate; MMF, mycophenolate mofetil.
The low-risk category included chronic myeloid leukemia in chronic phase, acute leukemia in first remission, myelodysplastic syndrome without excess blasts, and aplastic anemia. The high-risk category included all other diseases and stages.
Direct progression from acute GVHD to chronic GVHD or onset of chronic GVHD during steroid treatment.
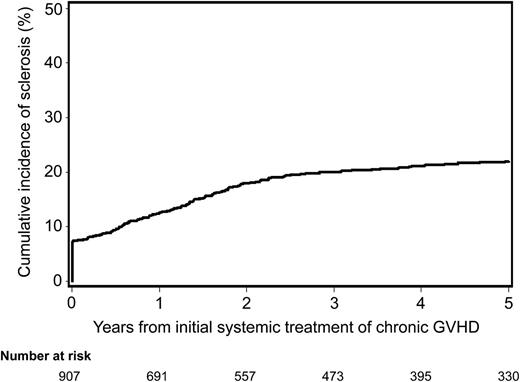
The cumulative incidence of sclerosis was 20% (95% confidence interval [CI], 17.5%–22.5%) at 3 years after initial systemic treatment for chronic GVHD (Figure 1). Seventy (7%) of the 977 patients had sclerosis at the time of initial treatment for chronic GVHD. The median time from initial systemic treatment for chronic GVHD to onset of sclerosis was 16.1 months (range, 0.1–92 months) among the 140 patients who had sclerosis after initial treatment. Overall, of the 210 patients who developed sclerosis, 28 (13%) had only skin sclerosis, 70 (33%) had only joint contracture or fasciitis, and 112 (53%) had both clinical manifestations.
Cumulative incidence of sclerosis after initial systemic treatment for chronic GVHD. Seventy (7%) of the 977 patients presented with sclerotic features at the time of initial systemic treatment for chronic GVHD.
Cumulative incidence of sclerosis after initial systemic treatment for chronic GVHD. Seventy (7%) of the 977 patients presented with sclerotic features at the time of initial systemic treatment for chronic GVHD.
Risk factors for sclerosis
In multivariate analyses (Table 2), we identified 4 patient or transplant-related factors associated with risk of sclerosis at any time among all patients with chronic GVHD. Factors associated with an increased risk of sclerosis included the use of a mobilized blood cell graft (HR 1.99; 95% CI, 1.20–3.31; P = .008) as compared with a bone marrow graft and TBI exposure >450 cGy in the conditioning regimen (HR 1.62; 95% CI, 1.14–2.31; P = .008) as compared with no TBI. Factors associated with a decreased risk of sclerosis included the use of an HLA-mismatched donor (HR 0.57; 95% CI, 0.37–0.89; P = .01) as compared with an HLA-matched donor and the use of a major ABO-mismatched donor (HR 0.65; 95% CI, 0.45–0.94; P = .02) as compared with an ABO-matched donor. Because the decreased risk of sclerosis associated with HLA mismatching was unexpected, further analyses were performed to determine whether HLA class I or class II mismatching was associated with a decreased risk of sclerosis. The HR was 0.64 (95% CI, 0.40–1.01; P = .06) for HLA class I mismatching and 0.36 (95% CI, 0.13–1.02; P = .06) for HLA class II mismatching. The test of homogeneity between class I and class II mismatching did not show statistical significance (P = .32).
Risk factors for sclerosis
| Risk factor . | Univariate . | Multivariate* . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Patient age Per 10 y | 1.10 (1.01–1.20) | .04 | ||
| Donor age Per 10 y | 1.07 (0.96–1.18) | .24 | ||
| Patient gender | ||||
| Male | 1.00 (reference) | |||
| Female | 1.25 (0.95–1.63) | .11 | ||
| Donor–recipient gender combination | ||||
| Male to male | 1.00 (reference) | |||
| Female to male | 0.70 (0.48–1.02) | .07 | ||
| Male to female | 0.87 (0.58–1.31) | .50 | ||
| Female to female | 1.22 (0.86–1.72) | .26 | ||
| Disease risk at transplantation | ||||
| Low | 1.00 (reference) | |||
| High | 1.11 (0.84–1.48) | .46 | ||
| Stem cell graft source | ||||
| Bone marrow | 1.00 (reference) | 1.00 (reference) | ||
| Mobilized blood cells | 2.15 (1.31–3.53) | .003 | 1.99 (1.20–3.31) | .008 |
| Umbilical cord blood | 0.57 (0.08–4.29) | .59 | 0.74 (0.10–5.63) | .77 |
| Donor relation | ||||
| Related | 1.00 (reference) | |||
| Unrelated | 0.98 (0.75–1.29) | .89 | ||
| HLA matching | ||||
| Matched | 1.00 (reference) | 1.00 (reference) | ||
| Mismatched | 0.51 (0.33–0.78) | .002 | 0.57 (0.37–0.89) | .01 |
| ABO compatibility | ||||
| Match | 1.00 (reference) | 1.00 (reference) | ||
| Minor mismatch | 1.08 (0.78–1.49) | .66 | 1.13 (0.81–1.57) | .46 |
| Major mismatch | 0.62 (0.43–0.90) | .01 | 0.65 (0.45–0.94) | .02 |
| Intensity of conditioning regimen | ||||
| High | 1.00 (reference) | |||
| Reduced | 1.26 (0.94–1.68) | .12 | ||
| TBI dose in conditioning regimen | ||||
| None | 1.00 (reference) | 1.00 (reference) | ||
| ≤450 cGy | 1.38 (1.01–1.89) | .05 | 1.27 (0.93–1.75) | .14 |
| >450 cGy | 1.40 (0.99–1.99) | .06 | 1.62 (1.14–2.31) | .008 |
| ATG in conditioning regimen | ||||
| No | 1.00 (reference) | |||
| Yes | 0.55 (0.26–1.17) | .12 | ||
| GVHD prophylaxis | ||||
| Cyclosporine + MTX/MMF | 1.00 (reference) | |||
| Tacrolimus + MTX/MMF | 1.14 (0.86–1.52) | .35 | ||
| Other | 0.71 (0.29–1.73) | .45 | ||
| Prior grade II–IV acute GVHD | ||||
| No | 1.00 (reference) | |||
| Yes | 0.87 (0.65–1.17) | .37 | ||
| Prior stage 3–4 skin acute GVHD | ||||
| No | 1.00 (reference) | |||
| Yes | 1.02 (0.77–1.35) | .88 | ||
| Risk factor . | Univariate . | Multivariate* . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Patient age Per 10 y | 1.10 (1.01–1.20) | .04 | ||
| Donor age Per 10 y | 1.07 (0.96–1.18) | .24 | ||
| Patient gender | ||||
| Male | 1.00 (reference) | |||
| Female | 1.25 (0.95–1.63) | .11 | ||
| Donor–recipient gender combination | ||||
| Male to male | 1.00 (reference) | |||
| Female to male | 0.70 (0.48–1.02) | .07 | ||
| Male to female | 0.87 (0.58–1.31) | .50 | ||
| Female to female | 1.22 (0.86–1.72) | .26 | ||
| Disease risk at transplantation | ||||
| Low | 1.00 (reference) | |||
| High | 1.11 (0.84–1.48) | .46 | ||
| Stem cell graft source | ||||
| Bone marrow | 1.00 (reference) | 1.00 (reference) | ||
| Mobilized blood cells | 2.15 (1.31–3.53) | .003 | 1.99 (1.20–3.31) | .008 |
| Umbilical cord blood | 0.57 (0.08–4.29) | .59 | 0.74 (0.10–5.63) | .77 |
| Donor relation | ||||
| Related | 1.00 (reference) | |||
| Unrelated | 0.98 (0.75–1.29) | .89 | ||
| HLA matching | ||||
| Matched | 1.00 (reference) | 1.00 (reference) | ||
| Mismatched | 0.51 (0.33–0.78) | .002 | 0.57 (0.37–0.89) | .01 |
| ABO compatibility | ||||
| Match | 1.00 (reference) | 1.00 (reference) | ||
| Minor mismatch | 1.08 (0.78–1.49) | .66 | 1.13 (0.81–1.57) | .46 |
| Major mismatch | 0.62 (0.43–0.90) | .01 | 0.65 (0.45–0.94) | .02 |
| Intensity of conditioning regimen | ||||
| High | 1.00 (reference) | |||
| Reduced | 1.26 (0.94–1.68) | .12 | ||
| TBI dose in conditioning regimen | ||||
| None | 1.00 (reference) | 1.00 (reference) | ||
| ≤450 cGy | 1.38 (1.01–1.89) | .05 | 1.27 (0.93–1.75) | .14 |
| >450 cGy | 1.40 (0.99–1.99) | .06 | 1.62 (1.14–2.31) | .008 |
| ATG in conditioning regimen | ||||
| No | 1.00 (reference) | |||
| Yes | 0.55 (0.26–1.17) | .12 | ||
| GVHD prophylaxis | ||||
| Cyclosporine + MTX/MMF | 1.00 (reference) | |||
| Tacrolimus + MTX/MMF | 1.14 (0.86–1.52) | .35 | ||
| Other | 0.71 (0.29–1.73) | .45 | ||
| Prior grade II–IV acute GVHD | ||||
| No | 1.00 (reference) | |||
| Yes | 0.87 (0.65–1.17) | .37 | ||
| Prior stage 3–4 skin acute GVHD | ||||
| No | 1.00 (reference) | |||
| Yes | 1.02 (0.77–1.35) | .88 | ||
ATG, antithymocyte globulin; MTX, methotrexate; MMF, mycophenolate mofetil.
Multivariate analysis includes risk factors with at least 1 category statistically significant at P = .05 level, 977 patients included.
In a separate analysis, we compared chronic GVHD-related characteristics at the time of initial systemic treatment for chronic GVHD between patients with and without sclerosis at the onset of systemic treatment. Eosinophilia was more frequently observed among the 70 patients with sclerosis as compared with the 907 patients without sclerosis at the onset of systemic treatment (46% vs 14%, P < .0001). Thrombocytopenia and progressive onset were less frequently observed among patients with sclerosis as compared with those without sclerosis (11% vs 34%, P < .0001 and 11% vs 36%, P < .0001, respectively). In a separate Cox model, we examined chronic GVHD-related characteristics at the time of initial systemic treatment as potential predictive factors for subsequent development of sclerosis among the 907 patients who did not have sclerosis at the onset of systemic treatment. None of the chronic GVHD-related characteristics were associated with the subsequent development of sclerosis.
Associations of sclerosis with transplant outcomes
Associations of sclerosis at any time with transplant outcomes were examined in all patients with chronic GVHD, treating development of sclerosis as a time-dependent risk factor (Table 3). Covariates associated with overall mortality in multivariate analyses were used for adjustment, including <5 months from HCT to initial systemic treatment for chronic GVHD as compared with ≥5 months, older patient age, high-risk disease status at HCT, and thrombocytopenia at initial systemic treatment for chronic GVHD. HLA mismatch was not associated with risk of overall mortality in multivariate analysis.
Association of sclerosis with transplant outcomes
| Outcome . | HR* (95% CI) . | P . |
|---|---|---|
| Overall mortality | 0.87 (0.63–1.19) | .37 |
| Nonrelapse mortality† | 1.00 (0.67–1.49) | .99 |
| Recurrent malignancy† | 0.85 (0.53–1.34) | .48 |
| Withdrawal of immunosuppressive treatment | 0.65 (0.50–0.85) | .001 |
| Outcome . | HR* (95% CI) . | P . |
|---|---|---|
| Overall mortality | 0.87 (0.63–1.19) | .37 |
| Nonrelapse mortality† | 1.00 (0.67–1.49) | .99 |
| Recurrent malignancy† | 0.85 (0.53–1.34) | .48 |
| Withdrawal of immunosuppressive treatment | 0.65 (0.50–0.85) | .001 |
Time-dependent analysis including all patients in the study cohort. Adjusted by months from transplantation to chronic GVHD, patient age, disease risk, and thrombocytopenia at time of initial systemic treatment for chronic GVHD.
Analyzed among 930 patients with malignancies.
Adjusted time-dependent Cox models showed that development of sclerosis was not associated with risks of subsequent overall mortality (HR 0.87; 95% CI, 0.63–1.19; P = .37), nonrelapse mortality (HR 1.00; 95% CI, 0.67–1.49; P = .99), or recurrent malignancy in patients with chronic GVHD (HR 0.85; 95% CI, 0.53–1.34; P = .48). Sclerosis was associated with a reduced rate of withdrawal of immunosuppressive treatment (HR 0.65; 95% CI, 0.50–0.85; P = .001), indicating that duration of immunosuppressive treatment was prolonged in chronic GVHD patients with sclerosis as compared with those without sclerosis.
Discussion
We found a substantial incidence of sclerosis after allogeneic HCT in a cohort of consecutive patients with chronic GVHD. The incidence of sclerosis reached 20% at 3 years after the initial systemic treatment for chronic GVHD; this was higher than the rates of 10% to 15% reported in previous studies.6,7 A single-visit cross-sectional study reported that the prevalence of sclerosis was 53% in a cohort of 206 patients with relatively severe chronic GVHD evaluated at the NIH Clinical Center.8 Our study showed that one third of patients with sclerosis presented with this clinical feature at the time of initial systemic treatment for chronic GVHD. The presence of sclerosis at initial treatment for chronic GVHD was associated with eosinophilia and, less frequently, thrombocytopenia, which is consistent with other reports.7,8 However, none of the chronic GVHD-related characteristics were associated with subsequent development of sclerosis.
We identified 4 patient or transplant-related factors associated with the risk of sclerosis among patients with chronic GVHD: use of a mobilized blood cell graft, conditioning with TBI >450 cGy, HLA mismatch, and ABO mismatch. The protective association of HLA mismatching was counterintuitive, given the positive association of HLA mismatching as a risk factor for chronic GVHD.15 The use of an unrelated donor, older patient age, use of a female donor for a male recipient, and prior acute GVHD were not identified as risk factors for sclerosis in the current study, although they are well-recognized risk factors for chronic GVHD.15-20 These results suggest that the profile of risk factors associated with sclerosis differs from the profile associated with chronic GVHD.
The protective effect of HLA mismatching was not caused by higher early mortality rates because HLA mismatching was not associated with overall mortality in our cohort. Moreover, early mortality due to competing risks does not affect the HR, which reflects the relative probability of mortality among patients actually at risk throughout observation. This consideration represents the primary motivation for using HR analysis, as opposed to analysis of incidence.21
Mechanisms that might explain the reduced risk of sclerosis associated with HLA mismatching remain speculative. For example, HLA mismatching could have an immunodominant effect that blocks the development of fibrogenic donor T-cell responses against “sclerogenic” minor antigens derived from connective tissues in the recipient. In fact, Amir et al reported that responses to minor histocompatibility antigens were not generated in the presence of stimulation by mismatched major histocompatibility (MHC) antigens after HLA-mismatched HCT.22 In murine models, cutaneous sclerosis has been reported as a manifestation of GVHD after MHC-identical, minor antigen-mismatched HCT with cells from B10-strain donors to certain BALB-strain recipients.23 Further work will be needed to confirm whether introduction of an MHC mismatching in these murine models abrogates the development of sclerosis.
Many studies have shown that the use of mobilized blood cell graft is associated with an increased risk of chronic GVHD.15,24 Our results show that the use of mobilized blood cell graft was also associated with an increased risk of sclerosis. This fits with a recent study that showed that stem cell mobilization with granulocyte colony-stimulating factor promoted murine sclerotic GVHD by inducing type 17 differentiation of T cells.25 In addition, associations of serum IL-17 concentrations with disease severity of systemic sclerosis were observed.26
Prior TBI exposure was more frequent among patients with sclerosis than among those without sclerosis in a cross-sectional study.8 Our study identified TBI exposure >450 cGy as a risk factor for sclerosis among patients with chronic GVHD. Irradiated skin is predisposed to cutaneous GVHD in humans.27 In a murine model, mice grafted with irradiated syngeneic skin and injected with allogeneic lymphocytes developed skin GVHD in the irradiated graft but not in nonirradiated grafts.28 Irradiation may upregulate expression of MHC molecules and interferon-γ production in the skin. These effects, combined with epidermal damage and systemic cytokine production, might increase the risk of sclerosis.
We expected that development of sclerosis could have negative associations with survival outcomes. However, overall mortality, nonrelapse mortality, and recurrent malignancy did not differ statistically between chronic GVHD patients with and without sclerosis, suggesting that sclerosis does not greatly affect the overall prognosis in patients with chronic GVHD. Sclerosis, nonetheless, is often associated with considerable disability and morbidity, and the prolonged duration of immunosuppressive treatment demonstrated in our study is likely to increase the risk of infections and other late complications.
Our study has some limitations. First, the incidence of sclerosis may be underestimated due to potential underreporting of sclerosis by local providers who may not be familiar with this manifestation of chronic GVHD. Second, available data did not allow further analyses regarding severity and extent of sclerosis, levels of disability, patient-reported parameters, quality-of-life measures, social recovery parameters, or any assessment of clinical response of sclerotic manifestations after treatment. Finally, our results require careful interpretation because of multiple comparisons. For example, the association of HLA mismatching with a decreased risk of sclerosis was counterintuitive and the association of major ABO mismatching with a decreased risk of sclerosis may be difficult to explain. Further studies are warranted to determine whether findings from the current study hold true.
In conclusion, we found a substantial incidence of sclerosis in patients with chronic GVHD. Our findings are useful for counseling patients about sclerosis after HCT and may help to identify candidates for early interventional studies aimed to reduce disability related to sclerosis. Furthermore, our findings will foster future studies of pathogenic mechanisms of sclerotic GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the National Institutes of Health (CA163438, CA18029, CA15704, CA100019, CA78902, HL36444, HL094260, AI033484, AI 041721, and AI069197). Y.I. is a recipient of the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad.
Authorship
Contribution: Y.I., P.J.M., and M.E.D.F. designed the study, collected and analyzed data, and wrote the report; B.E.S. performed the statistical analysis; E.W.P. reviewed donor–recipient HLA typing and assignment and wrote the report; S.J.L., P.A.C., and B.M.S. collected data and wrote the report; J.L.N. and J.A.H. contributed to interpretation of data and wrote the report. All authors approved the final report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary E. D. Flowers, Fred Hutchinson Cancer Research Center, D5-290, PO Box 19024, Seattle, WA 98109-1024; e-mail: mflowers@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal