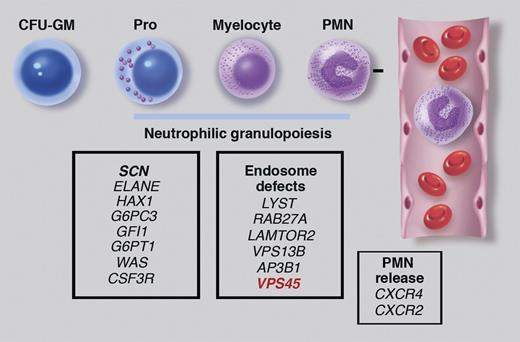
In this issue of Blood, Stepensky et al identify mutations of VPS45 as a new cause of congenital neutropenia and primary myelofibrosis.1
Genetic causes of congenital neutropenia. Neutrophilic granulopoiesis occurs primarily in the bone marrow through the stepwise maturation of myeloid progenitors (eg, colony-forming unit–granulocyte, monocyte, or CFU-GM) to promyelocytes (pro), myelocytes, and finally mature neutrophils (PMNs). PMNs are then released into the blood in a regulated fashion. Gene mutations associated with SCN are shown in the left panel. These mutations are typically associated with isolated severe neutropenia and a block in granulocytic differentiation. In contrast, mutations of CXCR4 and CXCR2 cause neutropenia by inhibiting neutrophil release from the bone marrow. Stepensky et al describe a new syndrome characterized by neutropenia, myelofibrosis, and impaired protein trafficking to endosomes that is due to VPS45 mutations. Mutations of VPS45 can be added to the list of gene mutations that affect protein trafficking to endosomes and are associated with congenital neutropenia (middle panel). Professional illustration by Marie Dauenheimer.
Genetic causes of congenital neutropenia. Neutrophilic granulopoiesis occurs primarily in the bone marrow through the stepwise maturation of myeloid progenitors (eg, colony-forming unit–granulocyte, monocyte, or CFU-GM) to promyelocytes (pro), myelocytes, and finally mature neutrophils (PMNs). PMNs are then released into the blood in a regulated fashion. Gene mutations associated with SCN are shown in the left panel. These mutations are typically associated with isolated severe neutropenia and a block in granulocytic differentiation. In contrast, mutations of CXCR4 and CXCR2 cause neutropenia by inhibiting neutrophil release from the bone marrow. Stepensky et al describe a new syndrome characterized by neutropenia, myelofibrosis, and impaired protein trafficking to endosomes that is due to VPS45 mutations. Mutations of VPS45 can be added to the list of gene mutations that affect protein trafficking to endosomes and are associated with congenital neutropenia (middle panel). Professional illustration by Marie Dauenheimer.
Severe congenital neutropenia (SCN) is characterized by severe isolated neutropenia from birth, recurring bacterial infections, and a marked propensity to develop a myelodysplastic syndrome or acute myeloid leukemia. SCN is a genetically heterogeneous disorder (see figure). Mutations of ELANE encoding neutrophil elastase (NE) account for ∼60% of cases (all in autosomal dominant or sporadic SCN). Mutations of HAX1, G6PC3, GFI1, G6PT1, WAS, and CSF3R collectively account for approximately 10% to 20% of cases of SCN. Thus, the genetic basis for approximately 20% to 30% of cases of SCN is unknown. Of note, all the aforementioned gene mutations are thought to induce neutropenia through the disruption of granulopoiesis. In contrast, mutations of CXCR4 or CXCR2 result in neutropenia through impaired release of neutrophils from the bone marrow to blood.
Stepensky et al1 describe 5 patients from 2 unrelated families with consanguineous marriages who presented with recurrent infection in the first year of life. They were found to have severe persistent neutropenia, progressive transfusion-dependent anemia, and variable thrombocytopenia. They also presented with features of myelofibrosis, including increased bone marrow fibrosis, teardrop red blood cells, and mild splenomegaly. Unlike most cases of SCN, maturation arrest in the myeloid series was not observed and the patients did not respond to granulocyte colony-stimulating factor. A single patient underwent allogeneic stem cell transplantation with correction of the hematopoietic defects, suggesting that VPS45 mutations act in a cell intrinsic fashion to disrupt hematopoiesis.
Using a combination of linkage analysis and whole exome sequencing, Stepensky et al1 identified homozygous Thr224Asn mutations of VPS45 in all 4 affected patients with available DNA. This mutation was not observed in nonaffected family members or in a large number of healthy individuals. VPS45 encodes for a protein that contributes to the assembly of the SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) complex. The SNARE complex plays an essential role in the trafficking of proteins to lysosomes and other endosomes in the cell. Prior studies in yeast have established that loss of VPS45 results in the degradation of key components of the SNARE complex and defective transport of proteins from the trans–Golgi network to endosomes.2 Stepensky et al1 provide strong evidence that the Thr224Asn VPS45 mutation is a loss-of-function mutation. First, they show that VPS45 protein expression is reduced in lymphocytes from patients homozygous for Thr224Asn VPS45. Second, they show, using complementation studies in yeast that lack endogenous Vps45, that wild-type, but not Thr224Asn, VPS45 restores protein trafficking to lysosomes.
While the mechanisms by which VPS45 deficiency leads to neutropenia and myelofibrosis are unclear, it is worth noting that loss-of-function mutations of genes implicated in protein trafficking to endosomes also are associated with congenital neutropenia, including LYST (Chédiak-Higashi syndrome); RAB27A (Griscelli syndrome); AP3B1 (Hermansky-Pudlak syndrome type 2); VPS13B (Cohen syndrome), and LAMTOR2 (see figure). Each of these disorders is characterized by neutropenia and variable degrees of platelet dysfunction, albinism, and cytotoxic T-cell dysfunction. The selective defects in immune cells, such as neutrophils and cytotoxic T cells, platelets, and melanocytes, are likely due, at least in part, to the extensive network of secretory lysosomes in these cells. Of note, lysosomes and α granules were reduced in fibroblasts and platelets, respectively, from patients homozygous for Thr224Asn VPS45.
This study raises several important questions. First, what is the frequency of VPS45 mutations in unselected patients with SCN? Although the Thr224Asn VPS45 mutation is associated with distinct clinical features (ie, myelofibrosis), it is possible that other VPS45 mutations may present with neutropenia alone. Second, what are the molecular mechanisms by which Thr224Asn VPS45 induces neutropenia and myelofibrosis? Similar to SCN associated with ELANE, HAX1, or G6PC3 mutations, Stepensky et al show that neutrophils from patients with Thr224Asn VPS45 display increased apoptosis. Although the evidence that Thr224Asn VPS45 disrupts protein sorting to lysosomes is convincing, it is not clear how this results in increased apoptosis. Of note, previous studies have demonstrated that loss-of-function mutations of AP3B1, which also disrupt protein sorting to lysosomes, result in impaired targeting of NE to primary granules and accumulation of NE precursor in the endoplasmic reticulum.3,4 Accumulation of misfolded mutant NE in the endoplasmic reticulum with subsequent induction of the unfolded protein response has been implicated in the pathogenesis of SCN associated with ELANE mutations.5,6 Thus, it is possible that impaired trafficking of NE (and other primary granule constituents) to granules in Thr224Asn VPS45 granulocytic precursors may disrupt granulopoiesis through the induction of the unfolded protein response.
VPS45 mutations can now be added to the growing list of gene mutations causing congenital neutropenia and should be considered in the differential diagnosis, especially for those children presenting with myelofibrosis. Studies to further define the molecular pathogenesis of this disorder will likely provide novel insights into the regulation of hematopoiesis and bone marrow fibrosis.
Conflict-of-interest disclosure: The author declares no competing financial interests.