To the editor:
The CD1 proteins are major histocompatibility complex class I-like molecules specialized in presenting lipid and glycolipid antigens to T cells.1 Group I CD1s include CD1a, CD1b, and CD1c and present bacterial antigens to a diverse repertoire of pathogen-specific T cells.2 In contrast, CD1d presents endogenous and exogenous ligands to invariant natural killer T cells.3 Different subsets of dendritic cells (DCs) express distinct CD1 profiles.4,5 Myeloid DCs (mDCs) in the blood express CD1d, whereas Langerhans cells in the skin express CD1a, suggesting that signals present in the microenvironment regulate these antigen presentation pathways. We have reported previously that IgG regulates the expression of CD1 molecules on human DCs in vitro, via an CD32a (FcγRIIa)–dependent mechanism.6 In the present study, we took advantage of the low IgG levels in treatment-naïve patients with common variable immunodeficiency (CVID) to investigate if IgG might regulate the CD1 expression profile also in vivo.7,8
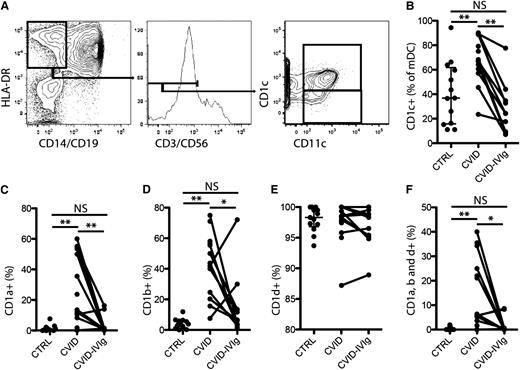
Peripheral blood mononuclear cells isolated from 13 patients newly diagnosed with CVID according to the criteria established by the Pan-American Group for Immunodeficiency before the onset of intravenous immunoglobulin (IVIg) replacement therapy were analyzed by flow cytometry. Approval was obtained from the University of Sao Paulo institutional review board for these studies, and informed consent was provided according to the Declaration of Helsinki. mDCs were defined as CD3-CD14-CD19-CD56-CD11c+HLA-DR+ (Figure 1A).9 The proportion of mDCs expressing CD1c was elevated in treatment-naïve patients with CVID compared with healthy control participants (Figure 1B). Interestingly, this subset bias was normalized after 12 months of IVIg treatment (Figure 1B). Next, we investigated the expression of CD1a, CD1b, and CD1d on the CD1c+ mDC subset. As expected, CD1a and CD1b were essentially undetectable on CD1c+ mDCs from healthy control participants (Figure 1C-D), whereas CD1d was ubiquitously expressed (Figure 1E). In contrast, CD1c+ mDCs from treatment-naïve patients with CVID expressed elevated levels of CD1a and CD1b (Figure 1C-D). Levels of CD1d were normal on the CD1c+ mDCs from patients with CVID (Figure 1E). Particularly striking was the abnormal presence of up to 40% of mDCs in some patients co-expressing CD1a, b, and d (Figure 1F). IVIg treatment significantly reduced the levels of CD1a and CD1b mono-expression, as well as CD1a, CD1b, and CD1d triple-expression in CD1c+ mDCs (Figure 1C-F). Expression levels of all 4 types of CD1 molecules on mDCs in patients with CVID receiving IVIg were not significantly different from those in healthy control participants, suggesting that the aberrant CD1 expression pattern can be normalized by raising the plasma levels of IgG.
Aberrant expression of CD1 molecules in mDCs in CVID is normalized by IVIg. (A) Representative staining-and-gating strategy. (B) Comparison of the level of CD1c+ mDCs between healthy control participants and patients with CVID, and in these patients before and after IVIg treatment. Comparison of the levels of CD1a expression (C); CD1b expression (D); CD1d expression (E); and CD1a, CD1b, and CD1d triple-expression (F) on CD1c+ mDCs between healthy control participants and patients with CVID, and in these patients before and after IVIg treatment. **P < .01 and *P < .05.
Aberrant expression of CD1 molecules in mDCs in CVID is normalized by IVIg. (A) Representative staining-and-gating strategy. (B) Comparison of the level of CD1c+ mDCs between healthy control participants and patients with CVID, and in these patients before and after IVIg treatment. Comparison of the levels of CD1a expression (C); CD1b expression (D); CD1d expression (E); and CD1a, CD1b, and CD1d triple-expression (F) on CD1c+ mDCs between healthy control participants and patients with CVID, and in these patients before and after IVIg treatment. **P < .01 and *P < .05.
Given the important role that CD1-mediated antigen presentation plays in both antigen-specific and immunoregulatory responses, it is likely that the CD1 antigen presentation pathways are regulated to provide suitable activation signals to the immune system. Our previous findings indicated that IgG is a mediator in the microenvironment that can influence the expression pattern of CD1 molecules in monocyte-derived DC cultures.6 The present results from patients with CVID indicate that mDCs express elevated levels of, in particular, CD1a and CD1b in the presence of low levels of IgG in vivo. Furthermore, this aberrant expression pattern is normalized after IVIg therapy, suggesting that IgG can regulate group I CD1 molecules in vivo in a similar manner to what we previously reported in vitro.
Authorship
Acknowledgments: The authors thank all patients and healthy donors for their time and effort toward this study.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, Stockholm County Council, Karolinska Institutet, and the US National Institutes of Health grant AI52731. D.P.-P. is the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research. B.A.N.S. is the recipient of a master fellowship from the Conselho Nacional de Pesquisa, Brazilian Ministry of Science and Technology. K.I.C. is the recipient of a postdoctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior through the Programa Nacional de Pós Doutorado, Brazilian Ministry of Education. The group at the University of São Paulo coordinates one of the Brazilian National Institutes of Science and Technology (Instituto de Investigação em Imunologia – iii), funded by the CNPq.
Contribution: D.P.-P. designed the research, performed experiments, analyzed data, and co-wrote the paper; B.A.N.S. designed the research, performed experiments, analyzed data, and provided samples; K.I.C. and M.M. designed the research; M.T.-B., A.K.B.d.O., C.M.K., and J.K. provided samples; and E.G.K. and J.K.S. designed the research, analyzed data, and co-wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johan K. Sandberg, Center for Infectious Medicine, Department of Medicine, F59, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm 14186, Sweden; e-mail: johan.sandberg@ki.se.
References
Author notes
D.P.-P., B.A.N.S., E.G.K., and J.K.S. contributed equally to this work.