In this issue of Blood, Gandre-Babbe et al have, in part, overcome the obstacle of validating the molecular underpinnings of juvenile myelomonocytic leukemia (JMML) with the generation of induced pluripotent stem cells (iPSCs) from individuals with JMML.1
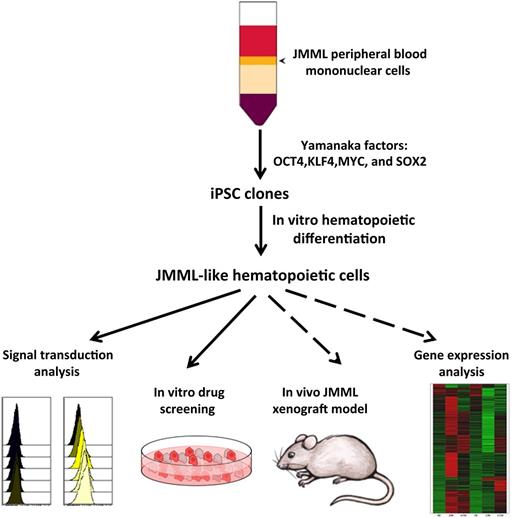
Peripheral blood or bone marrow cells from patients with JMML were infected with a lentiviral vector expressing an inducible construct of all four Yamanaka factors. Induced iPSC clones were identified, and selected clones were differentiated into hematopoietic cells by using established protocols. Cells with JMML-like phenotypic and functional qualities were detected through several assays, including signal transduction analysis and responsiveness to growth factors. These cells were responsive to chemicals that inhibit certain signaling pathways. In future studies, these JMML-like cells can be tested for in vivo engraftment in immunodeficient mouse models and for more detailed molecular analysis.
Peripheral blood or bone marrow cells from patients with JMML were infected with a lentiviral vector expressing an inducible construct of all four Yamanaka factors. Induced iPSC clones were identified, and selected clones were differentiated into hematopoietic cells by using established protocols. Cells with JMML-like phenotypic and functional qualities were detected through several assays, including signal transduction analysis and responsiveness to growth factors. These cells were responsive to chemicals that inhibit certain signaling pathways. In future studies, these JMML-like cells can be tested for in vivo engraftment in immunodeficient mouse models and for more detailed molecular analysis.
Validation of the molecular underpinnings of JMML derived from mouse models,2 has been limited in humans due to a scarcity of primary clinical samples. JMML is a rare childhood leukemia that has nonetheless played a central and highly informative role in clarifying the consequences of Ras hyperactivation in human malignancies as well as in human congenital disorders.3 JMML also continues to be the recipient of intense investigation because its only curative therapy is allogeneic hematopoietic stem cell (HSC) transplantation, and yet 50% of children will succumb to leukemia relapse following this arduous therapy.3
JMML is renowned for displaying features of a disease based on well-defined and unambiguous genetic mutations, since most children diagnosed with JMML bear nonoverlapping loss-of-function mutations in NF1 or CBL or gain-of-function mutations in NRAS, KRAS, or PTPN11. Each of these mutations yields RAS hyperactivation with a net output of excessive signaling through RAS effector pathways, including the canonical RAF-MEK-ERK and PI3K-AKT pathways. Functionally, the hallmark of hematopoietic progenitors from individuals with JMML is cytokine-independent growth in vitro and exquisite sensitivity to the growth factor granulocyte macrophage colony-stimulating factor (GM-CSF).4
The advent of iPSC technology has provided an opportunity to generate a renewable source of patient-derived reagents that may potentially produce authentic models of JMML in vitro and in vivo for deeper molecular analysis, straightforward and efficient screening of novel pharmacologic agents, and refined genotype-dependent treatment recommendations (see figure). Thus, Gandre-Babbe et al1 have successfully generated iPSC clones from two individual JMML patients bearing the somatic PTPN11 mutation p.E76K (see figure). Multiple iPSC clones from each patient sample yielded increased myeloid cell (CD45+CD18+) production upon in vitro hematopoietic differentiation and produced increased myeloid colonies compared with wild-type controls. Consistent with the well-documented phenotype of primary JMML samples, the JMML iPSC-derived hematopoietic cells demonstrated cytokine-independent colony growth, hypersensitivity to GM-CSF, and signal transducer and activator of transcription 5 (STAT5) hyperphosphorylation in response to low GM-CSF concentrations. Initial pharmacologic studies demonstrated sensitivity of the JMML iPSC-derived hematopoietic cells to the MEK inhibitor PD0325901 (see figure), consistent with previous studies performed in loss-of-function Nf1 and gain-of-function Kras murine models.5 Collectively, these phenotypic qualities support the premise that these JMML iPSC clones can be used to generate hematopoietic progenitors that closely simulate primary JMML samples.
Although this work offers an exciting new tool in the armamentarium of JMML reagents, it also highlights unresolved questions in the field. First, it is notable that both published iPSC clones are derived from JMML samples harboring the PTPN11 mutation p.E76K. JMML patients with PTPN11 mutations are reported to have a significantly lower overall survival compared with patients with wild-type PTPN11. Furthermore, among PTPN11 mutations, the p.E76K mutation produces a protein with the highest known basal and unregulated tyrosine phosphatase activity among PTPN11 oncogenic protein products. In contrast, anecdotes of mild and spontaneously resolving disease have been reported for JMML patients with somatic NRAS and KRAS mutations.3 Therefore, one wonders if the successful origination of JMML-derived iPSC clones is unique to mutations bearing the strongest oncogenic activity, or if this technology will be able to be applied more broadly to samples carrying less deleterious mutations.
Second, generation of mutant hematopoietic progenitors with JMML-like features from differentiated iPSCs raises an intriguing question about the embryonic origin of the disease. Although the stem cell theory of hematopoiesis predicts that all blood cell lineages are derived via a stem cell precursor, recent information in the developing mouse suggests that erythromyeloid progenitor cells and B-1–cell and T-cell subsets arise during development prior to HSC emergence.6 Furthermore, some long-lived resident macrophage populations in the adult murine liver, skin, and brain are derived from embryonic macrophage precursors and are maintained life-long, seemingly independent of HSC contributions.7,8 Given the ongoing lack of proof for the generation of HSCs from differentiated human iPSCs9 the production of mutant hyperproliferative JMML-like myeloid progenitor cells by Gandre-Babbe et al1 suggests that these cells may have arisen from a non-HSC hemogenic endothelial-derived erythromyeloid progenitor cells. This is an intriguing hypothesis that serves as a new paradigm for understanding the ultimate origin of JMML disease in human patients. Since essentially half of the patients treated with allogeneic stem cell transplantation for JMML relapse, one wonders if the stem cells for this disease reside in tissue-resident monocyte-macrophage precursors and not in traditional HSC-derived myeloid progenitor cells. Essentially nothing is known about the location, composition, or function of the tissue macrophage niches that may exist. If some of the long-lived replenishing macrophage precursors are deeply quiescent, total body irradiation and/or chemotherapy may not penetrate and eliminate these cells. Evidence for long-lived tissue-resident macrophage progenitor cells that are resistant to total body irradiation has been reported.10 Development of methods to engraft the human iPSC-derived JMML-like cells in an optimized immunodeficient mouse model system (see figure) may assist in examining some of these exciting new questions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal