Key Points
RAF1 Ser259 phosphorylation is a critical regulator step controlling arterial morphogenesis and arterial-venous patterning.
ERK activation controls DLL4/Notch signaling and semaphorin 6A–mediated endothelial cell repulsion.
Arterial morphogenesis is one of the most critical events during embryonic vascular development. Although arterial fate specification is mainly controlled by the Notch signaling pathway, arterial-venous patterning is modulated by a number of guidance factors. How these pathways are regulated is still largely unknown. Here, we demonstrate that endothelial activation of RAF1/extracellular signal-regulated kinase (ERK) pathway regulates arterial morphogenesis and arterial-venous patterning via Δ/Notch and semaphorin signaling. Introduction of a single amino acid RAF1 mutant (RAF1 Ser259Ala), which renders it resistant to inhibition by phosphorylation, into endothelial cells in vitro induced expression of virtually the entire embryonic arteriogenic program and activated semaphorin 6A–dependent endothelial cell–cell repulsion. In vivo, endothelial-specific expression of RAF1S259A during development induced extensive arterial morphogenesis both in the yolk sac and the embryo proper and disrupted arterial-venous patterning. Our results suggest that endothelial ERK signaling is critical for both arteriogenesis and arterial-venous patterning and that RAF1 Ser259 phosphorylation plays a critical role in preventing unopposed ERK activation.
Introduction
Formation of arterial conduits, whether during development or in adult tissues, is a complex set of processes involving interactions between numerous factors. Although some details have been revealed, much remains to be learned about how arteries form and how a distinct arterial identity is established. Recent studies have suggested that there are fundamental molecular differences between the arterial and venous vasculatures and that arterial fate is determined early in the course of development, even before the onset of blood circulation in some settings.
Vascular endothelial growth factor (VEGF)-A is thought to play a crucial role in the establishment of arterial identity and induction of expression of specific arterial markers Notch1 and Δ-like 4 (DLL4) in endothelial cells (EC) in vitro and in vivo. Notch signaling is one of the master controls of arterial fate and is essential for both embryonic and postnatal arterial development.1,,,,,-7 Of the 5 Notch ligands- DLL1, 3, and 4, and JAG 1 and 2, DLL4 is of particular interest in arteriogenesis because of its specific role in tip cell formation8,-10 and embryonic arterial development.1,11 DLL4 acts in a dose-dependent manner,1,11 indicating that the precise control of its expression is vital for vascular development.
In the endothelium, VEGF-A signals primarily via VEGFR2, although it may also signal via VEGFR3.12,-14 One of the major signaling pathways activated by both VEGF receptors is the PLCγ-cPKC-RAF1-MEK-ERK pathway, which is tightly controlled by phosphorylation of its critical regulatory kinase RAF1. The regulation of RAF1 activity is a complex process that involves multiple phosphorylation events. Phosphorylation of certain RAF1 residues such as Ser621, Ser338, Tyr341, Thr491, and Ser494 increases its kinase activity, whereas phosphorylation of others such as Ser43, Ser233, and Ser259 decreases it.15 Among these, Ser259 plays a particularly critical role in RAF1 activation of downstream mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling. Phosphorylation of this site recruits protein 14-3-3 resulting in inactivation of RAF1, whereas dephosphorylation releases 14-3-3, leading to RAF1 activation.16 Ser259 can be phosphorylated by AKT17 and several other kinases including protein kinas A18 and protein kinase Cα,19 and is dephosphorylated by PP2A20.
Another major downstream target of VEGF signaling is the phosphatidylinositol 3-kinase (PI3K)-AKT pathway that is involved in angiogenic sprouting, vascular maturation, and regulation of permeability.21,22 It is also thought to be involved in venous fate specification due, in part, to its ability to suppress ERK activation by phosphorylating RAF1 Ser259 site. Importantly, inhibition of PI3K signaling has been reported to rescue arterial defects in zebrafish gridlock mutants presumably via removal of Akt-mediated inhibition of Raf1, thus leading to activation of Erk.23 Similarly, inhibition of PI3K activity restored arterial morphogenesis in mice with reduced VEGFR2 and ERK activation and defective arterial morphogenesis.24 Thus, VEGF activates interacting signaling pathways that regulate both arterial and venous differentiation.
In addition to establishing proper arterial-venous identities, formation of a fully functional circulatory network requires development of highly stereotyped vascular patterns. These include exquisite parallel alignment of arterial and venous networks25 that requires precise control over their guidance and boundary formation between arteries and veins. Factors controlling vascular patterning are not yet completely known. Proper establishment of vessel identity, which can be defined by a set of markers, such as ephrinB226 and Dll41,4,11 for arteries and EphB426 and neuropilin-227 for veins, is intrinsically linked to the patterning process.28 Deletion of these genes leads to vascular remodeling defects in the yolk sac vessels.1,2,26 In addition, several attractive and repulsive pathways are also known to modulate proper blood vessel guidance. These include the semaphorins, the netrins, the slits, the ephrins and their receptors.25,29,-31 However, how these molecules are regulated is still unknown.
In this study, we sought to determine the role of RAF1-dependent ERK activation in arterial morphogenesis during early mouse embryonic development. We found that blocking inhibition of RAF1 activation by preventing phosphorylation of Ser259 site in the endothelium leads to constitutive ERK activation that, in turn, results in induction of almost the entire arterial morphogenic gene expression program, including activation of DLL4-Notch signaling pathway. This resulted in formation of extensively branched arterial tree with enlarged lumens. In addition, endothelial RAF1S259A expression also led to an abnormal arterial-venous patterning characterized by large distances between arteries and veins likely resulting from increased endothelial cell repulsion mediated by the induction of semaphorin 6A (SEMA6A) expression.
Materials and methods
Antibodies and reagents
Antibodies used were p-ERK1/2, ERK1/2, RAF1, pRAF1 S259, cleaved NOTCH1, NOTCH1, and DLL4 (Cell Signaling); HA (Covance); pS6K T389, S6K (Epitomics); VE-Cadherin (Santa Cruz); CD31 (BD Biosciences); Connexin 40 (CX40) (Alpha Diagnostic); β-gal (Abcam); and smooth muscle actin (Sigma). U0126 was from Cell Signaling; α-tubulin (12G10) antibody was prepared from mice ascites fluid using a hybridoma from the Developmental Studies Hybridoma Bank. LY294002 and DAPT (N-[(3,5-Difluorophenyl)acetyl]-l-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester) were from Sigma. Fibronectin was from R&D.
Transgenic mice
Generation of TRE- RAF1S259A mice was performed as previously described.32 In this study, animals never received tetracycline. Timed matings were set up by crossing male TRE-RAF1S259A mice with VE-cadherin-tTA female mice. For paraffin and frozen sections, embryos were fixed in 4% paraformaldehyde (PFA) overnight at 4°C.
Immunohistochemistry
Hematoxylin and eosin staining was performed as previously described.33 Immunofluorescent staining of paraffin sections (7 μm) was performed by incubating primary and secondary antibodies in 0.5% Blocking Reagent (Perkin Elmer) buffered with 50 mM Tris-HCI pH 7.5, 150 mM NaCl and 0.05% Tween-20. Sections were then mounted using ProLong Gold antifade reagent from Invitrogen and imaged.
Whole mount immunofluorescent staining
Embryonic tissues were fixed in 4% PFA overnight at 4°C then washed 3× with phosphate-buffered saline (PBS). Tissues were permeabilized and blocked overnight at 4°C in TNBT buffer (100 mM Tris-HCl, pH7.5, 150 mM NaCl, 0.5% Triton-X100, and 0.05% Blocking Reagent) and washed with TNT buffer (100 mM Tris-HCl, pH7.5, 150 mM NaCl, 0.5% Triton-X100) 6× at room temperature. Tissues were then stained with primary antibodies overnight at 4°C followed by fluorescent secondary antibodies in TNBT for 2 hours at room temperature. After washing 6× with TNT buffer, tissues were mounted with ProLong Gold (Invitrogen) and imaged.
Image analysis
Hematoxylin and eosin images were acquired with a Nikon Eclipse 80i upright microscope. Fluorescent images were acquired using either a Leica SP5 confocal microscope or a Perkin Elmer spinning disk confocal microscope. To image whole mount immunofluorescent stained samples, z-stack images with a 1-μm step size were acquired on a Leica SP5 confocal microscope using LAS AF software. Vessel branching and artery size were analyzed using Angiotool and Image J software, respectively.
cDNA microarray and quantitative reverse-transcription PCR and western blot
Total RNA was purified using an RNeasyPlus Mini kit (Qiagen). RNA samples from 3 independent infections were used for complementary DNA (cDNA) microarray analysis using an Illumina HumanHT-12 V3.0 expression bead chip at the W.M. Keck Biotechnology Resource Laboratory, Yale University. A difference higher than 1.5-fold and false discovery rate < 0.05 compared with control cells was considered as significant. For quantitative polymerase chain reaction (qPCR), cDNA was synthesized using the iScript cDNA Synthesis kit (Bio-Rad). qPCR was performed using iQ SYBR Green Supermix (Bio-Rad) on a Bio-Rad CFX96 Real Time System. Primers used for qPCR are listed in supplemental Table 1. For western blotting, cells were lysed in radio-Immunoprecipitation assay buffer (150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) supplemented with protease and phosphatase inhibitors. Cell lysates were then separated with 4% to 20% gradient gel (Bio-Rad) and subjected to western blot. Densitometry of western blots was performed using Image J (NIH).
Primary mouse EC isolation
Primary mouse ECs were isolated from mouse embryos using a previously described protocol.24 Briefly, embryos were harvested, minced finely with scissors, and then digested in 25 mL collagenase 0.2% (wt/vol) at 37°C for 20 minutes. The crude cell preparation was pelleted, resuspended in Dulbecco’s PBS and then incubated with CD31-coated Dynabeads (Invitrogen) at 4 °C for 10 minutes with rotation. Using a magnetic separator, the bead-bound cells were recovered and washed with Dulbecco’s modified Eagle medium-20% fetal bovine serum. The cells were then used for gene expression analysis by qPCR.
Administration of U0126, LY294002, and DAPT
Cells were treated with U0126 (10 μg/mL), LY294002 (10 μg/mL), and DAPT (20 μmol/L) for 24 hours. Dimethylsulfoxide (DMSO) was used as the control.
Repulsion assay
Human umbilical vein endothelial cells (HUVECs) infected with pLVX-mCherry lentivirus were plated and grown to confluence on fibronectin coated glass-bottom plates (MatTek). HUVECs infected with adenovirus coexpressing RAF1 wild type (WT) or S259A and green fluorescence protein (GFP) were then plated on top of the mCherry cells and incubated for 4–6 hours. The cells were then washed 3 times with warm PBS and fixed with 4% PFA for 10 minutes at room temperature. Z-stack images were then acquired using either a Leica SP5 confocal microscope or a Perkin Elmer spinning disk confocal microscope with a 0.5-μm step size. Three-dimensional projections were generated using either Volocity 6.0 or LAS AF software. Ten randomly selected areas were imaged for each sample. Cell repulsion was quantified by calculating the overlapping area of green and red cells using Image J software (NIH). The percent overlap between red and green cells was used to represent the cell repulsion level and thus a higher percentage reflects more overlap and less repulsion between cells.
Data analysis
Differences between 2 groups were tested for statistical significance with a 2-tailed Student t test using SigmaPlot 11.0. A P value smaller than .05 was considered significant.
Accession numbers
The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE42964.
Study approval
All the protocols and experiments performed in mice were approved by the institutional animal care and utilization committee at Yale University School of Medicine, New Haven, CT.
Results
Blockade of RAF1 phosphorylation on Ser259 induces arteriogenesis and disrupts arterial/venous patterning
To determine the specific role played by deactivation of RAF1 signaling induced by Ser259 phosphorylation in blood vessel morphogenesis, we introduced a mutation into RAF1 Ser259 site that prevents its phosphorylation by AKT and other kinases (RAF1S259A, supplemental Figure 1A). As previously reported,32 expression of RAF1S259A in EC leads to activation of ERK, whereas overexpression of WT RAF1 has marginal effect on ERK activation (supplemental Figure 1B), suggesting that this effect was specifically caused by the blockade of RAF1 activation of Ser259 phosphorylation.
To further determine the role of RAF1 Ser259 in vivo, we generated an endothelial-specific RAF1S259A transgenic mouse (S259A) line using a Tet-off system.32 Modest expression of RAF1S259A in the yolk sac was confirmed by western blotting (supplemental Figure 1C-D). We have previously reported that endothelial expression of S259A transgene leads to embryonic lethality at embryonic day (E)15.5 because of lymphatic and cardiac defects that become prominent after E14.5, whereas major arteries in the embryo proper were largely unaffected.32 To analyze the effect of RAF1 activation on blood vasculature in these mice, we investigated vascular development at earlier developmental stages before the onset of severe lymphatic and cardiac abnormalities.
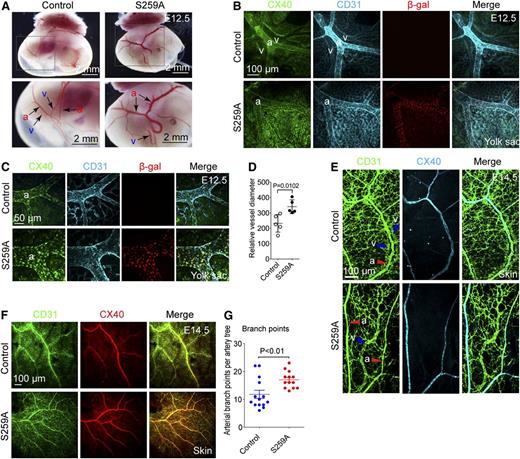
At E10.5, there were no significant differences in either embryonic or yolk sac vasculature between S259A transgenic and littermate single transgene or WT mice (data not shown). Direct visual examination of the yolk sac vasculature demonstrated that by E12.5 posterior vitelline arteries of S259A embryos were markedly enlarged and had significantly more branches than controls, whereas posterior vitelline veins were much smaller and sometimes invisible (Figure 1A) . This was confirmed by immunostaining of the yolk sac vasculature (Figure 1B-C).
Endothelial expression of RAF1S259A causes defective arterial morphogenesis. (A) Dark field images of posterior E12.5 yolk sacs with embryo inside. Higher magnification images of the areas highlighted in the square are shown in the lower panels. Posterior vitelline arteries (a) and veins (v) are indicated with arrows. Bar represents 2 mm. (B-C) Whole mount immunofluorescent staining of E12.5 yolk sacs. Arteries are stained with the arterial marker CX40. Bars represent 100 μm (B) and 50 μm (C). (D) Quantification of posterior vitelline artery size as shown in (C). The diameter of the 2 major branches of posterior vitelline arteries in each yolk sac was quantified and averaged to represent that of 1 embryo. Mean ± SEM. n = 5 embryos. (E) Whole mount immunofluorescent staining of E14.5 skin. Bar represents 100 μm. a, artery; v, vein. (F) Whole mount immunofluorescent staining of E14.5 skin. Bar represents 100 μm. (G) Quantitative analysis of arterial branching in skin of E14.5 embryos as shown in (F). Branch points of 4 major artery trees in each dorsal skin of an embryo were counted and averaged to represent that of one artery tree. Mean ± SEM. Control, n = 14 embryos; S259A, n = 13 embryos.
Endothelial expression of RAF1S259A causes defective arterial morphogenesis. (A) Dark field images of posterior E12.5 yolk sacs with embryo inside. Higher magnification images of the areas highlighted in the square are shown in the lower panels. Posterior vitelline arteries (a) and veins (v) are indicated with arrows. Bar represents 2 mm. (B-C) Whole mount immunofluorescent staining of E12.5 yolk sacs. Arteries are stained with the arterial marker CX40. Bars represent 100 μm (B) and 50 μm (C). (D) Quantification of posterior vitelline artery size as shown in (C). The diameter of the 2 major branches of posterior vitelline arteries in each yolk sac was quantified and averaged to represent that of 1 embryo. Mean ± SEM. n = 5 embryos. (E) Whole mount immunofluorescent staining of E14.5 skin. Bar represents 100 μm. a, artery; v, vein. (F) Whole mount immunofluorescent staining of E14.5 skin. Bar represents 100 μm. (G) Quantitative analysis of arterial branching in skin of E14.5 embryos as shown in (F). Branch points of 4 major artery trees in each dorsal skin of an embryo were counted and averaged to represent that of one artery tree. Mean ± SEM. Control, n = 14 embryos; S259A, n = 13 embryos.
In addition, although vitelline arteries and veins were aligned parallel to each other in yolk sacs of WT or single transgene mice, in S259A yolk sacs, vitelline veins were frequently located at a considerable distance from the artery or were absent altogether (Figure 1A-B). Similar arterial/venous patterning defects were observed in the skin of E14.5 and E15.5 embryos (Figure 1E and supplemental Figure 2). Furthermore, arteries in E14.5 S259A embryos were also more branched compared with that in control embryos (Figure 1F-G), whereas the capillary bed was significantly decreased in size in both E14.5 skin and E12.5 yolk sac (supplemental Figure 3).
RAF1 activation induces arterial gene expression
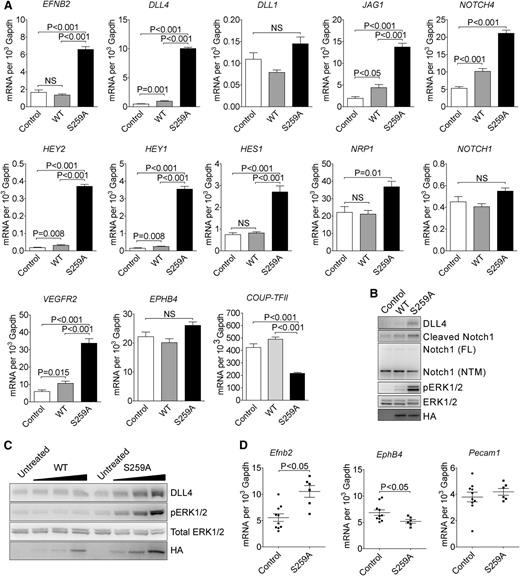
To further determine the role of RAF1 S259 phosphorylation in arterial morphogenesis, we performed gene expression analysis using cDNA microarray. The gene expression patterns in control and RAF1 WT expressing EC were almost identical, whereas expression of S259A transgene induced a significant shift in expression (supplemental Figure 4A). When compared with RAF1 WT cells, S259A expression upregulated 72 and downregulated 35 genes (supplemental Figure 4B). Strikingly, almost the entire embryonic arterial fate program, including arterial specific genes such as EFNB2, NRP1, DLL4, and NOTCH4 were dramatically upregulated as were downstream Notch pathway genes including HEY2, HEY1, HES1, and HES2. At the same time, venous markers were unchanged (EPHB4) or decreased (COUP-TFII) (Figure 2A).
RAF1S259A induces expression of the entire embryonic arteriogenic program. (A) qPCR of indicated genes in HUVECs infected with empty control, WT HA-RAF1 or HA-RAF1S259A (S259A) lentiviruses. Mean ± SEM. n = 3. (B) Immunoblot of control, RAF1 WT, and S259A lentivirus-infected HUVECs. (C) Immunoblot of RAF1 WT and S259A adenoviruses-infected HUVECs. (D) qPCR of E10.5 yolk sacs. Control, n = 10 embryos; S259A, n = 6 embryos. Mean ± SEM. HA, hemagglutinin; mRNA, messenger RNA; NS, not significant.
RAF1S259A induces expression of the entire embryonic arteriogenic program. (A) qPCR of indicated genes in HUVECs infected with empty control, WT HA-RAF1 or HA-RAF1S259A (S259A) lentiviruses. Mean ± SEM. n = 3. (B) Immunoblot of control, RAF1 WT, and S259A lentivirus-infected HUVECs. (C) Immunoblot of RAF1 WT and S259A adenoviruses-infected HUVECs. (D) qPCR of E10.5 yolk sacs. Control, n = 10 embryos; S259A, n = 6 embryos. Mean ± SEM. HA, hemagglutinin; mRNA, messenger RNA; NS, not significant.
Key array results were confirmed by qPCR analysis (Figure 2A). Of those upregulated genes, DLL4 is of particular interest because of its critical roles in artery formation and regulation of branching.8,-10 At the same time, expression of DLL1, a homolog of DLL4 that is also specifically expressed in arteries and is thought to be involved in arteriogenesis,5,10 was not affected.
In agreement with qPCR results, western blot analysis confirmed increased DLL4 and activation of NOTCH signaling in S259A-expressing EC (Figure 2B). Finally, transient transduction of HUVEC with RAF1S259A adenovirus led to a dose-dependent increase in DLL4 expression and ERK activation, whereas transduction with a WT RAF1 virus had only a marginal effect (Figure 2C).
In addition, qPCR analysis of E12.5 yolk sac demonstrated increased expression of Efnb2 and reduced expression of EphB4, whereas Pecam1 level was unchanged (Figure 2D).
ERK activity is required for arterial gene expression
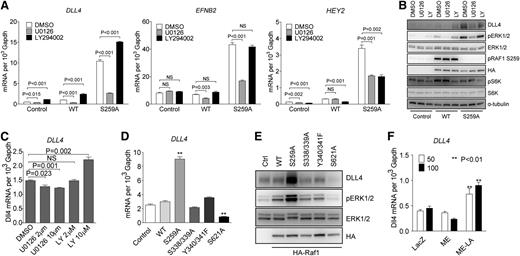
To further define ERK vs PI3K signaling contribution to activation of arterial gene expression, HUVECs were transduced with either RAF1 WT or RAF1S259A lentiviral constructs. In agreement with previous results, RAF1S259A but not RAF1 WT construct induced a large increase in DLL4, EFNB2, and HEY2 expression that was inhibited by treatment with a MEK inhibitor U0126 (Figure 3A). In contrast, treatment with a PI3K inhibitor LY294002 increased arterial markers’ expression in control (empty vector) or RAF1 lentivirus-transduced cells and even led to a further increase in expression in RAF1S259A lentivirus-transduced HUVECs (Figure 3A). Western blotting confirmed these results and demonstrated effective suppression of ERK signaling by U0126 (as shown by inhibition of pERK1/2) and PI3K by LY294002 (as shown by inhibition of pS6K) (Figure 3B). Noticeably, both DLL4 mRNA and protein levels perfectly correlated with the level of ERK activation (Figure 3A-B). Treatment of HUVEC with different doses of U0126 and LY294002 resulted, respectively, in a dose-dependent decrease (U0126) or increase (LY294002) in DLL4 expression (Figure 3C). In agreement with these results, VEGF-A– and VEGF-C–induced DLL4 expression were abolished by treatment with U0126 and enhanced by treatment with LY294002 (supplemental Figure 5).
RAF1 controls arterial gene expression via ERK signaling. (A) Effect of MEK and PI3K inhibition on arterial gene expression. HUVECs transduced with control, WT RAF1 or RAF1S259A lentiviruses were treated with DMSO, MEK inhibitor U0126 (10 μM), or PI3K inhibitor LY294002 (10 μM) for 24 hours. DLL4, EFNB2, and HEY2 expression was assessed by qPCR. Data represent mean ± SEM of 3 independent experiments. (B) Immunoblot of Control, RAF1 WT and S259A lentivirus-infected HUVECs were treated with DMSO, MEK inhibitor U0126, or PI3K inhibitor LY294002. (C) DLL4 qPCR of HUVECs treated with DMSO, MEK inhibitor U0126, or PI3K inhibitor LY294002 at indicated doses. (D) qPCR of HUVECs infected with control, WT RAF1, S259A, S338/339A, Y340/341F, or S621A lentiviruses. (E) Immunoblot of HUVECs infected with indicated lentiviruses. (F) DLL4 qPCR of HUVECs infected with ad-lacz, ME, or ME-LA adenoviruses. *P < .05; **P < .01. mRNA, messenger RNA; NS, not significant.
RAF1 controls arterial gene expression via ERK signaling. (A) Effect of MEK and PI3K inhibition on arterial gene expression. HUVECs transduced with control, WT RAF1 or RAF1S259A lentiviruses were treated with DMSO, MEK inhibitor U0126 (10 μM), or PI3K inhibitor LY294002 (10 μM) for 24 hours. DLL4, EFNB2, and HEY2 expression was assessed by qPCR. Data represent mean ± SEM of 3 independent experiments. (B) Immunoblot of Control, RAF1 WT and S259A lentivirus-infected HUVECs were treated with DMSO, MEK inhibitor U0126, or PI3K inhibitor LY294002. (C) DLL4 qPCR of HUVECs treated with DMSO, MEK inhibitor U0126, or PI3K inhibitor LY294002 at indicated doses. (D) qPCR of HUVECs infected with control, WT RAF1, S259A, S338/339A, Y340/341F, or S621A lentiviruses. (E) Immunoblot of HUVECs infected with indicated lentiviruses. (F) DLL4 qPCR of HUVECs infected with ad-lacz, ME, or ME-LA adenoviruses. *P < .05; **P < .01. mRNA, messenger RNA; NS, not significant.
To further confirm the specific effect of ERK activation on RAF1S259A-induced DLL4 expression, HUVECs were transduced with RAF1 mutants other than S259A. Inactivation of 2 fibroblast growth factor– (S338/339) or VEGF-dependent (Y340/341) phosphorylation sites affected neither ERK activation nor DLL4 expression. However, mutation of S621, a critical site for RAF1 activation, dramatically decreased both ERK activation and DLL4 expression (Figure 3D-E). Finally, to verify direct ERK dependence of DLL4 expression, HUVECs were transduced with a nuclear-localized constitutive active ERK (ME-LA) or the same ERK construct without nuclear localization signal (ME). ME-LA, but not ME, induced a significant increase in DLL4 expression (Figure 3F), indicating that a nuclear translocation of activated ERK is essential for DLL4 induction.
Constitutive RAF1 activation induces SEMA6A-dependent endothelial cell repulsion
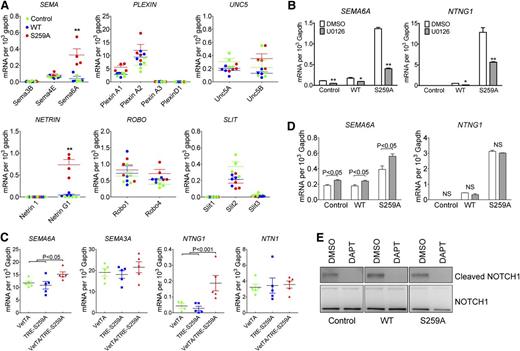
As shown previously, constitutive RAF1 activation in vivo induced by introduction of a Ser259 mutation to alanine disrupted proper arterial-venous alignment with veins being pushed away from their normal para-arterial position. The regulation of arterial-venous alignment is poorly understood but is thought to be controlled, in part, by various guidance molecules. To investigate if changes in ERK signaling affected expression of major guidance molecules (semaphorins, plexins, UNC5, netrins, ROBOs, and SLITs) in RAF1S259A expressing HUVECs, we used cDNA microarray (supplemental Figure 6) and qPCR analysis (Figure 4A). Two guidance molecules SEMA6A and Netrin G1 (NTNG1) were significantly upregulated in RAF1S259A cells (supplemental Figure 6 and Figure 4A). The same two genes were also significantly upregulated in primary EC isolated from E12.5 S259A embryos (Figure 4C). Inhibition of ERK signaling by a MEK inhibitor U0126 abolished SEMA6A and NTNG1 induction by RAF1S259A indicating an essential role of ERK signaling in regulating SEMA6A and NTNG1 (Figure 4B).
RAF1S259A induces SEMA6A and NTNG1 expression. (A) qPCR analysis of guidance signaling pathways in HUVECs infected with control, WT RAF1, or RAF1S259A lentiviruses. Data represent mean ± SEM of 4 independent experiments. *P < .05; **P < .01. (B) Effect of MEK and PI3K inhibition on SEMA6A and NTNG1 expression. HUVEC transduced with control, WT RAF1, or RAF1S259A lentiviruses were treated with DMSO, MEK inhibitor U0126 (10 mM) for 24 hours. Gene expression was assessed by qPCR. Data represent mean ± SEM of 3 independent experiments. (C) qPCR analysis of guidance signaling pathways in primary ECs isolated from E12.5 embryos. n = 5 embryos. (D) Effect of NOTCH inhibition on SEMA6A and NTNG1 gene expression was assessed by qPCR. HUVECs transduced with control, WT RAF1, or RAF1S259A lentiviruses were treated with DMSO or DAPT for 24 hours. Data represent mean ± SEM of 3 independent experiments. (E) Immunoblot of HUVECs treated with DAPT.
RAF1S259A induces SEMA6A and NTNG1 expression. (A) qPCR analysis of guidance signaling pathways in HUVECs infected with control, WT RAF1, or RAF1S259A lentiviruses. Data represent mean ± SEM of 4 independent experiments. *P < .05; **P < .01. (B) Effect of MEK and PI3K inhibition on SEMA6A and NTNG1 expression. HUVEC transduced with control, WT RAF1, or RAF1S259A lentiviruses were treated with DMSO, MEK inhibitor U0126 (10 mM) for 24 hours. Gene expression was assessed by qPCR. Data represent mean ± SEM of 3 independent experiments. (C) qPCR analysis of guidance signaling pathways in primary ECs isolated from E12.5 embryos. n = 5 embryos. (D) Effect of NOTCH inhibition on SEMA6A and NTNG1 gene expression was assessed by qPCR. HUVECs transduced with control, WT RAF1, or RAF1S259A lentiviruses were treated with DMSO or DAPT for 24 hours. Data represent mean ± SEM of 3 independent experiments. (E) Immunoblot of HUVECs treated with DAPT.
Because Notch signaling is also induced by RAF1S259A, we then asked whether SEMA6A and NTNG1 induction is due to increased Notch signaling. Inhibition of Notch signaling by a γ-secretase inhibitor DAPT (Figure 4D-E) either had no effect on NTNG1 or slightly increased SEMA6A level, indicating that Notch signaling is not involved in RAF1S259A-induced SEMA6A and NTNG1 expression.
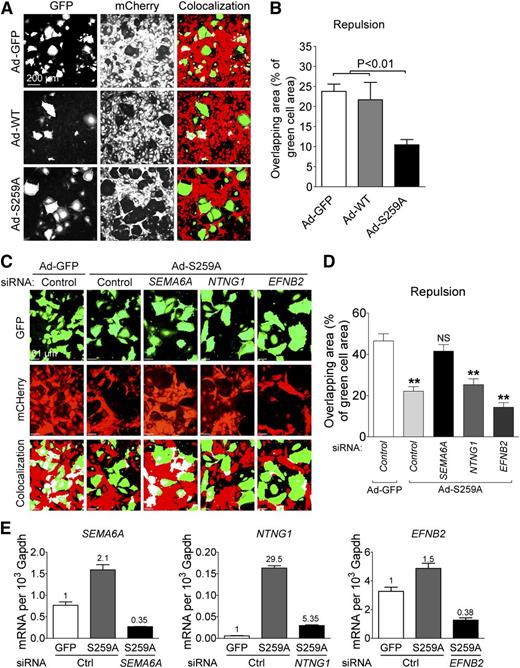
Semaphorins and netrins are known to regulate direct cell-to-cell-communication and can induce repulsive signals. A recent study showed that SEMA6A controls EC repulsion.34 In addition, EFNB2 was also shown to regulate EC repulsion.35 Given that RAF1S259A induces SEMA6A, NTNG1, and EFNB2, we expected that RAF1S259A would induce EC repulsion as well. To test this hypothesis, HUVECs transduced with adenoviruses expressing GFP alone or together with RAF1 WT or RAF1S259A were plated on top of confluent HUVECs transduced with lentiviral mCherry construct. Cell repulsion was determined 4–6 hours later by measuring appearance of green cells among the red cell monolayer. Transduction with Ad-RAF1S259A but not RAF1 resulted in a significant increase in repulsion (Figure 5A-B). To test whether SEMA6A, NTNG1, and EFNB2 contribute to the repulsive effect of RAF1S259A, these genes were knocked down in RAF1S259A cells. Silencing SEMA6A but not the other 2 genes significantly decreased EC repulsion induced by RAF1S259A (Figure 5C-E).
RAF1S259A induces SEMA6A-dependent EC repulsion. (A) Repulsion assay of HUVECs infected with ad-GFP, RAF1 WT, and S259A. Green cells are HUVECs infected with ad-GFP, RAF1 WT, and S259A and transfected with indicated small interfering RNAs (siRNAs). Red cells are HUVEC infected with mCherry lentivirus. Colocalized areas were indicated as white. (B) Quantification of repulsion assay shown in (A). Data represent mean ± SEM of 3 independent experiments. (C) Repulsion assay of HUVECs after knocking down SEMA6A, NTNG1, and EFNB2. (D) Quantification of repulsion assay shown in (C). Data represent mean ± SEM of 3 independent experiments. Knocking down efficiency is shown in (E). mRNA, messenger RNA.
RAF1S259A induces SEMA6A-dependent EC repulsion. (A) Repulsion assay of HUVECs infected with ad-GFP, RAF1 WT, and S259A. Green cells are HUVECs infected with ad-GFP, RAF1 WT, and S259A and transfected with indicated small interfering RNAs (siRNAs). Red cells are HUVEC infected with mCherry lentivirus. Colocalized areas were indicated as white. (B) Quantification of repulsion assay shown in (A). Data represent mean ± SEM of 3 independent experiments. (C) Repulsion assay of HUVECs after knocking down SEMA6A, NTNG1, and EFNB2. (D) Quantification of repulsion assay shown in (C). Data represent mean ± SEM of 3 independent experiments. Knocking down efficiency is shown in (E). mRNA, messenger RNA.
Discussion
Arterial morphogenesis is a complex process that occurs both during embryonic development and in adult tissues in response to a variety of signals such as organ growth, ischemia, and tissue injury. Despite its obvious importance, little is known about the responsible intracellular signaling cascades. It involves multiple events such as arterial fate specification, branching, and patterning. Although VEGF signaling is thought to play a central role in arterial morphogenesis, the precise sequence of events remains undefined. Previously several studies from our laboratory have shown that synectin-mediated VEGFR2-dependent ERK activation is required for effective arterial morphogenesis.24,36,37 However, many details of this signaling pathway are still unclear. In this study, we addressed the role played by RAF1 in regulation of ERK activation in arterial morphogenesis during early mouse embryonic development. To this end, we generated a RAF1 mutant resistant to phosphorylation on a Ser259 site that leads to suppression of ERK signaling. The expression of this mutant construct in EC in vitro or in vivo leads to a constitutive activation of ERK signaling. Thus, activated ERK induced expression of almost the entire arterial morphogenesis program, including a full activation of DLL4/Notch signaling cascade, leading to formation of extensively branched arterial network with larger than normal lumens. In addition, we observed increased separation between arteries and veins resulting from increased EC repulsion mediated by ERK-induced SEMA6A expression.
DLL4/Notch signaling is one of the major determinants of arterial fate.1,6,11,38 Our results suggest that RAF1-dependent ERK activation plays a key role in regulating Notch signaling by controlling the expression of its ligands DLL4 and JAG. Interestingly, there was no significant change in expression of DLL1 that has been implicated in both adult and embryonic arteriogenesis.5,10 EC express both Notch 1 and Notch 4. The expression of the latter but not the former was also increased by persistent ERK activation. This induction of both Notch receptors and ligands led to a profound increase in Notch activation, including increased expression of a number of Notch-dependent transcription factors such as HEY2, HEY1, and HES1. The functional counterpart of this increase in Notch activity was a significant enlargement of arteries in yolk sacs of S259A embryos, whereas veins appeared retarded—possibly signifying a venous to arterial identity shift. Interestingly, dorsal aorta and cardinal veins in the embryo proper were largely unaffected at early embryonic stages. This could be due to the relative lower expression level of RAF1S259A transgene in embryos compared with that in yolk sacs at these stages of development or could be a sign of intrinsic differences in vascular properties between embryo and yolk sac, which is reflected by a common notion that the vasculature of yolk sac is often more susceptible to genetic alterations than that of the embryo proper.
The arterial tree, both in the yolk sac and in the embryo proper, also appeared more extensive and with larger lumens. These findings are consistent with previous reports of reduced complexity of arterial networks and smaller lumen diameters in mice with decreased VEGFR2-dependent ERK activation. Thus, knockout of synectin and myosin-VI, proteins involved in VEGFR2 endocytic trafficking, results in reduced ERK activation, and mice homozygous for deletion of either gene demonstrate reduced arterial morphogenesis.36,37
In addition to inducing arterial fate specification, DLL4/Notch signaling also plays a critical role in vascular sprouting and branching as a negative regulator. In agreement with this aspect of Notch function, branching of the capillary bed was significantly decreased in S259A embryos (supplemental Figure 3).
VEGF regulation of DLL4 expression is a matter of controversy. VEGFR2 activates both PI3K-AKT and PLCγ-RAF-MEK-ERK pathways, and both pathways have been implicated in regulation of DLL4 expression.23,39,-41 However, although ERK was reported to regulate Dll4 in zebrafish,23 it was found to be dispensable in mammals.39,40 In our studies, inhibition of ERK almost completely abolished RAF1S259A-induced DLL4 expression, whereas inhibition of PI3K-AKT pathway led to an increased in DLL4 levels. In addition, if PI3K-AKT pathway induces DLL4, inhibition of this pathway would reduce arteriogenesis. In contrast to that prediction, in vivo studies from our laboratory and others showed that inhibiting PI3K-AKT pathway induces rather than reduces arteriogenesis.24,41 Furthermore, RAF1S259A induces expression not only of DLL4 but also of other genes downstream of DLL4, such as EFNB2 and HEY2 in an ERK-dependent manner, indicating a critical role of ERK signaling in DLL4 expression. Nevertheless, our results do indicate a role of PI3K-AKT pathway in Notch signaling because its inhibition leads to decreased HEY2 level, suggesting that this gene’s expression requires both ERK and PI3K signaling input.
Another interesting observation in this study was the loss of arterial-venous alignment from persistent ERK activation. Gene array studies identified SEMA6A, a member of the semaphorin gene family, as a likely culprit. This was confirmed by in vitro repulsion assays in which a knockdown of SEMA6A expression induced by RAF1S259A in HUVECs reduced cell repulsion to control levels. Although semaphorins were originally thought to mediate axon guidance during the development of central nervous system,42 a number of the family members such as SEMA3A, SEMA3E, SEMA3F, SEMA4D, and SEMA6A are expressed in EC and play various roles in endothelium.43,-45 In particular, SEMA3E30,43 and SEMA6A45 have been implicated in regulating vascular patterning and EC repulsion, and this is supported by our data.
Alternatively, vascular patterning can be also affected by changes in vessel identity.1,2,26,46 EFNB2, an arterial marker that is upregulated by RAF1S259A both in vitro and in vivo, has been reported to regulate EC repulsion.35 However, silencing EFNB2 had no effect on RAF1S259A-induced cell repulsion, indicating that this mechanism cannot account for the abnormal arterial-venous patterning observed in our studies.
Prior studies have identified PI3K/ERK cross-talk as a regulator of arterial morphogenesis by demonstrating that suppression of PI3K signaling can restore arteriogenesis in zebrafish or mice mutants with defective arterial development.24,41 The current study provides specific evidence that phosphorylation of RAF1 Ser259 site is the key element in the regulation of ERK activation and ERK-dependent arterial morphogenesis. ERK is activated when Ser259 is not phosphorylated; this is required for arterial morphogenesis in general and, specifically, for activation of Δ/Notch signaling. However, a prolonged unopposed ERK activation, resulting from a lack of RAF1 Ser259 phosphorylation, leads to a decrease in capillary bed sprouting and abnormal arterial-venous patterning likely from increased endothelial expression of SEMA6A.
Although AKT is the most obvious kinase that can regulate RAF1/ERK signaling in this way, it is not the only family of kinases that can phosphorylate RAF1 Ser259 site, nor can a role of a Ser/Thr phosphatase in this process be excluded. Furthermore, the timing and duration of RAF1 Ser259 phosphorylation are just as important as the nature of the kinase or the phosphatase involved. These related questions are a matter for ongoing studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Guri Tzivion (Wayne State University) for RAF1 constructs, Dr Laura Benjamin (Harvard Medical School) for VE-cadherin-tTA mice, Yan Huang (Yale) for preparing adenoviruses, Rita Webber and Nicole Copeland (Yale) for expert mouse care, Aiping Lin (Yale) for cDNA microarray analysis, and Dr Anthony Lanahan (Yale) for manuscript editing.
This work was supported in part by National Institutes of Health grants HL084619 and HL107205 (M.S.) and a Leducq Foundation Transatlantic Network grant (Y.D., A.E., M.S.).
Authorship
Contribution: Y.D., A.E., and M.S. designed experiments; Y.D., B.L., Z.W.Z., D.A., F.M., and C.P. performed experiments; Y.D., A.E., and M.S. interpreted data; and Y.D. and M.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Simons, Section of Cardiovascular Medicine, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06510; e-mail: michael.simons@yale.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal