Key Points
An adjuvant (alum), known to boost immune responses, can be used to facilitate a tolerogenic protocol.
Nondepleting anti-CD4 can lead to Foxp3+ regulatory T-cell–independent tolerance that relies on IL-10.
Current treatment of hemophilia consists of the administration of recombinant clotting factors, such as factor VIII (FVIII). However, patients with severe hemophilia can mount immune responses targeting therapeutically administered FVIII through inhibitory immunoglobulins that limit treatment efficacy. Induction of immune tolerance to FVIII in hemophilia has been extensively studied but remains an unmet need. We found that nondepleting anti-CD4 monoclonal antibodies (mAbs) are effective in inducing long-term tolerance to FVIII in different strains of hemophilic mice. Tolerance induction was facilitated when anti-CD4 mAbs were administered together with FVIII adsorbed in an adjuvant (alum). The observed state of tolerance was antigen specific, with mice remaining immune competent to respond to different antigens. Importantly, we found that following immunization with FVIII, the primed cells remained susceptible to tolerance induction. Studies with Foxp3-deficient and interleukin 10 (IL-10)–deficient mice demonstrated that the underlying tolerance mechanism is Foxp3 independent but requires IL-10. Our data show that an adjuvant, when administered together with a tolerizing agent such as nondepleting anti-CD4, can facilitate the induction of long-term tolerance to recombinant proteins, possibly not only in hemophilia but also in other diseases that are treated with potentially immunogenic therapeutics.
Introduction
For many patients with severe hemophilia, replacement therapy with recombinant clotting factors represents an immune challenge with nonself proteins, thus resulting in an immune response. As a consequence, immunoglobulins targeting infused clotting factors can lead to their inhibition (those immunoglobulins are termed inhibitors).1 Several approaches for immune tolerance induction (ITI), leading to the control of the production of clotting factor inhibitors, are currently used in hemophilia patients.2 However, the success of ITI is not absolute, and currently, at a time when viral contaminants in therapeutic products are a problem of the past, inhibitors have become a major complication for hemophilia treatment. This has led to an increasing interest in developing new protocols for the induction of tolerance to therapeutically administered clotting factors.3 Recent clinical trials have explored the putative higher tolerogenicity of factor VIII (FVIII) coupled with von Willebrand factor or B-cell depletion with the anti-CD20 monoclonal antibody (mAb) rituximab.4,5 The outcome of those trials has shown, at the most, a modest improvement in ITI efficacy. Consequently, those ITI approaches were not generally adopted, and the development of better strategies for ITI remains an unmet medical need.6
In recent years, several strategies have been developed to promote immune tolerance toward alloantigens. Several studies have demonstrated that nondepleting mAbs targeting T-cell surface molecules are effective in achieving immune tolerance in transplantation and autoimmunity.7,8 There is, however, a contrast between the efficacy in achieving long-term tolerance with mAbs such as CD4 or CD40L administered at the time of transplantation in preclinical studies9,-11 and a transient effect observed when the same reagents are used to induce tolerance to human recombinant FVIII (hFVIII) or factor IX in mice.12,13 Remarkably, when the recombinant clotting factors are expressed in tissues, for instance following viral transduction, tolerance induction appears to be easier to achieve.14
These observations prompted us to investigate whether the difference between both outcomes is a consequence of differences in the way the antigen is presented to T cells for tolerance induction. It is plausible that efficient imposition of tolerance in vivo would require an impact on a large proportion of the antigen-specific T cells or stronger costimulation, something that can only be achieved in the presence of inflammation (namely in transplantation or viral transduction) or when an adjuvant is used.
We found that the most clinically relevant adjuvant, aluminum hydroxide (alum), facilitates the induction of tolerance to hFVIII in mice. Surprisingly, the mechanism underlying tolerance induction appears to be different from antibody-induced transplantation tolerance; although nondepleting anti-CD4 induces Foxp3+ regulatory T (Treg) cells that are indispensable for transplantation tolerance, we were able to induce tolerance to foreign proteins in Foxp3-deficient mice. On the contrary, tolerance induction is critically dependent on interleukin 10 (IL-10), as it cannot be established in IL-10−/− mice.
Adjuvants can therefore have 2 opposing roles: they can contribute to boost immune responses and to overcome natural tolerance, but they can also be instrumental in facilitating therapeutically induced tolerance when in the presence of the appropriate tolerance-inducing reagents.
Materials and methods
Animals
BALB/c, C57Bl/6, B6.IL-10−/−, B6.Rag2−/−, and DO11.10.Rag2−/− mice were maintained under specific pathogen-free conditions. Hemophilia A mice (HemA) with a targeted disruption of exon 16 in the FVIII gene,15 backcrossed into C57Bl/6 and BALB/c background, were provided by Professor David Lillicrap (Kingston, Canada). T/B monoclonal mice (T-Bmc, 17/9 DO11.10.RAG1−/−) bear monoclonal T and B lymphocytes specific for ovalbumin (OVA) 323-339 and hemagglutinin (HA) of influenza virus, respectively.16 Procedures were approved by Direção Geral de Veterinária (Lisbon).
Animals were exposed to 1 U hFVIII (Kogenate, Bayer, Germany) or to 20 µg OVA (Sigma, St Louis, MO) sometimes adsorbed in 2.0 mg endotoxin-free aluminum hydroxide (Alu-gel-S, Serva, Heidelberg, Germany).
Monoclonal antibodies
Nondepleting anti-CD4 (YTS177.9),17 the isotype control (YKIX302), anti-CD25 (PC61), anti–IL-10 receptor (IL-10R) (1B1.1), and anti–transforming growth factor β (TGF-β) (1D11) were produced in-house.
Quantification of immunoglobulins and cytokines
hFVIII-specific, OVA-specific, and HA-specific immunoglobulin G1 (IgG1) were measured by enzyme-linked immunosorbent assay using IgG1 horseradish peroxidase (SouthernBiotech, Birmingham, AL) with anti-hFVIII IgG1 standard (Abcam, Cambridge, UK), anti-OVA IgG1 standard (Serotec, Oxford, UK), and anti-HA IgG1 standard (Sigma). The enzyme-linked immunosorbent assay for IL-10 measurement was performed using a kit (Peprotech, London, UK).
Flow cytometry
Cells were stained with CD25-PE-Cy7 (PC61.5), Foxp3-APC (FJK-16s), CD3-PE-Cy7 (145-2C11), CD4-PerCp (RM4-5), and DO11.10 T-cell receptor–specific KJ1-26-PE. Six color analyses were performed using an LSR-Fortessa (BD Bioscience).
Bethesda assay
Inhibitory FVIII antibodies were measured using the Bethesda assay and reported as Bethesda units (BU) per milliliter as previously described.18 Briefly, mouse plasma was serially diluted (Technochrom FVIII:C; Technoclone, Austria) such that the residual FVIII:C for each sample was between 25% and 75%, mixed 1:1 with pooled normal human plasma (FVIII inhibitor reagent kit), and incubated for 2 hours at 37°C. The remaining FVIII activity was quantified using a chromogenic assay (Technochrom). To reduce error in the assay and inconsistency between samples, the reported inhibitor titer in BU per milliliter was calculated from the dilution of plasma that yielded a residual FVIII:C of approximately 50%. By definition, 1 BU/mL is the dilution of plasma containing FVIII inhibitory activity that results in 50% inhibition of FVIII activity (FVIII:C).
Statistical analysis
Statistical significance was determined using the 2-tailed nonparametric Mann-Whitney U test, and values of P < .05 were deemed significant.
Results
hFVIII alum increases the tolerogenicity of anti-CD4 mAb
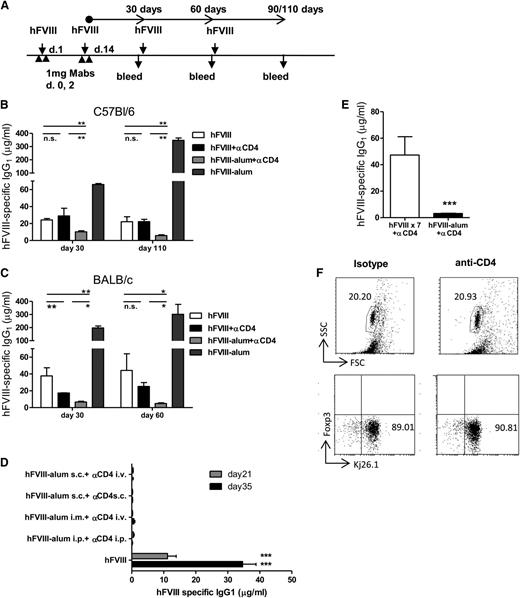
Alum has been used as an adjuvant in the formulation of human vaccines since 1926.19,20 We treated BALB/c and C57Bl/6 mice with hFVIII or hFVIII-alum together with nondepleting anti-CD4 at the indicated time points (Figure 1A). Serum anti-hFVIII IgG1 was measured following additional administrations of hFVIII. Our results confirmed that anti-CD4 treatment only has a moderate impact in preventing the generation of inhibitor antibodies to hFVIII (Figure 1B-C). However, when hFVIII-alum was used at the time of anti-CD4 treatment, the concentration of hFVIII-specific antibodies remained significantly reduced, even following multiple administrations of hFVIII, up to 110 days following the initial treatment. Note that hFVIII-alum is, as anticipated, highly immunogenic and leads to high levels of anti-hFVIII immunoglobulins when used in the absence of anti-CD4. We also quantified the hFVIII-specific immunoglobulin G2a and immunoglobulin G2b that remained negligible in both strains (supplemental Figure 1).
hFVIII-alum increases the tolerogenicity of anti-CD4 mAb. (A) C57Bl/6 and BALB/c mice were treated with 1 U hFVIII or hFVIII-alum and 2 × 1 mg nondepleting anti-CD4 (or isotype control) on the indicated days, followed by subsequent administrations of 1 U hFVIII as represented. The presence of serum hFVIII-specific immunoglobulin was investigated at days 30, 60, and 90 or 110 following the last administration of anti-CD4. (B-C) Serum concentration of hFVIII-specific IgG1 in C57Bl/6 mice (B) (n = 6; **P < .01) and in BALB/c mice (C) (n = 6; *P < .05; **P < .01). (D) hFVIII-alum and anti-CD4 were administered through different routes (as indicated in the figure label) according to the schedule represented in panel A. One group of mice was treated with an isotype control. All mice were then exposed to hFVIII intravenously and serum anti-hFVIII IgG1 was quantified. Animals that received hFVIII-alum together with anti-CD4 remained tolerant regardless of the administration route (n = 6; ***P < .001). (E) Persistent serum levels of hFVIII did not prevent the generation of hFVIII-specific IgG1 in the presence of anti-CD4. C57Bl/6 mice were treated with anti-CD4 mAbs in the days indicated above. Some mice were treated with 1 U hFVIII every other day for 2 weeks. We found that animals exposed to multiple administrations of hFVIII still produced higher titers of anti-hFVIII IgG1 than animals treated with hFVIII-alum (n = 6; ***P < .001). (F) Administration of the nondepleting anti-CD4 mAb (YTS177.9) does not lead to CD4 T-cell depletion. DO11.10.Rag−/− mice were treated with 2 × 1 mg YTS177.9, and splenocytes were examined by flow cytometry for evidence of T-cell depletion at day 7 (n = 5; P = nonsignificant). i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; n.s., nonsignificant; s.c., subcutaneous.
hFVIII-alum increases the tolerogenicity of anti-CD4 mAb. (A) C57Bl/6 and BALB/c mice were treated with 1 U hFVIII or hFVIII-alum and 2 × 1 mg nondepleting anti-CD4 (or isotype control) on the indicated days, followed by subsequent administrations of 1 U hFVIII as represented. The presence of serum hFVIII-specific immunoglobulin was investigated at days 30, 60, and 90 or 110 following the last administration of anti-CD4. (B-C) Serum concentration of hFVIII-specific IgG1 in C57Bl/6 mice (B) (n = 6; **P < .01) and in BALB/c mice (C) (n = 6; *P < .05; **P < .01). (D) hFVIII-alum and anti-CD4 were administered through different routes (as indicated in the figure label) according to the schedule represented in panel A. One group of mice was treated with an isotype control. All mice were then exposed to hFVIII intravenously and serum anti-hFVIII IgG1 was quantified. Animals that received hFVIII-alum together with anti-CD4 remained tolerant regardless of the administration route (n = 6; ***P < .001). (E) Persistent serum levels of hFVIII did not prevent the generation of hFVIII-specific IgG1 in the presence of anti-CD4. C57Bl/6 mice were treated with anti-CD4 mAbs in the days indicated above. Some mice were treated with 1 U hFVIII every other day for 2 weeks. We found that animals exposed to multiple administrations of hFVIII still produced higher titers of anti-hFVIII IgG1 than animals treated with hFVIII-alum (n = 6; ***P < .001). (F) Administration of the nondepleting anti-CD4 mAb (YTS177.9) does not lead to CD4 T-cell depletion. DO11.10.Rag−/− mice were treated with 2 × 1 mg YTS177.9, and splenocytes were examined by flow cytometry for evidence of T-cell depletion at day 7 (n = 5; P = nonsignificant). i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; n.s., nonsignificant; s.c., subcutaneous.
Next, we investigated whether the route of hFVIII-alum administration could influence tolerance induction. C57Bl/6 mice were exposed to hFVIII-alum intraperitoneally (as described above), intramuscularly, or subcutaneously. Subsequent administrations of hFVIII were performed intravenously (Figure 1D). We found that tolerance to hFVIII could be induced regardless of the route of administration.
The “depot effect” has been regarded as a possible mechanism by which alum exerts its adjuvanticity.21 According to this effect, adsorption of hFVIII in alum would confer a slow release and consequently an increased half-life of hFVIII in vivo, thus providing stable and long-term antigen presentation. This hypothesis is especially relevant as it has been described that sustained release of peptides, by an implanted osmotic pump, can lead to immune tolerance.22 We addressed this issue by repeatedly injecting hFVIII every other day for 2 weeks in the presence of anti-CD4 mAb. We found that animals exposed to multiple administrations of hFVIII plus anti-CD4 still produced higher titers of anti-hFVIII IgG1 than animals treated with hFVIII-alum plus anti-CD4 (Figure 1E).
Although the anti-CD4 mAb has a nondepleting isotype, we confirmed it does not lead to direct T-cell depletion in vivo by treating DO11.10.Rag2−/− mice with anti-CD4. In this way, we could circumvent the coating of the CD4 molecule and were able to accurately quantify CD4+ T cells by staining their transgenic T-cell receptor (identified with KJ26.1 mAbs). We found that the frequency of CD4+ splenocytes at day 7 following anti-CD4 treatment remained unchanged (Figure 1F).
Tolerance induction requires IL-10 and is independent of Foxp3+ Treg cells
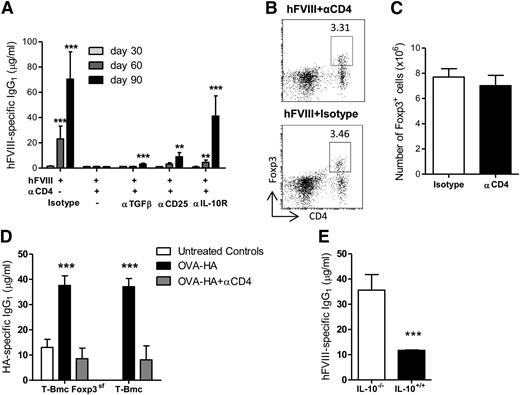
We and others have shown that transplantation tolerance can be induced with nondepleting anti-CD4 mAb, in a process that is Foxp3+ Treg-cell mediated and TGF-β dependent.9,23,,-26 In some conditions, IL-10 appears to play a role in transplantation tolerance.24,27 In order to study the mechanism by which anti-CD4 mAb induces tolerance in hFVIII-alum–treated animals, we used mAbs to block TGF-β or IL-10R at the time of treatment. In addition, another group of animals was treated with depleting anti-CD25 mAb prior to treatment. We found that IL-10R blockade during the initial 2 weeks had a dramatic effect in preventing tolerance induction (Figure 2A). However, only a modest increase in anti-hFVIII IgG1 was observed in mice subjected to depletion of CD25+ T cells or to neutralization of TGF-β.
Tolerance induction requires IL-10 and is independent of Foxp3+ Treg cells. (A) Mice were treated with hFVIII-alum and anti-CD4 as described in Figure 1A. Different groups were also treated with the indicated mAbs. All mice were exposed to 1 U hFVIII on days 30 and 60. Serum concentrations of hFVIII-specific IgG1 were determined on days 30, 60, and 90 (n = 5; **P < .01; ***P < .001). (B-C) Representative dot-plots (B) and quantification of splenic CD4+CD25+Foxp3+ T cells (C) from animals treated with anti-CD4 mAb or an isotype control on day 90 (n = 5; P = nonsignificant). (D) T/B monoclonal mice (T-Bmc) and T-Bmc Foxp3-deficient mice (T-Bmc FoxP3sf) were immunized on days 1 and 14 with OVA-HA-alum in the presence of nondepleting anti-CD4 or an isotype control. Serum HA-specific IgG1 was determined on day 24 (n = 5; ***P < .001). (E) IL-10−/− and IL-10+/+ mice were treated with hFVIII-alum and anti-CD4 mAbs as described above. The serum concentration of hFVIII-specific IgG1 was quantified on day 90 (n = 6; ***P < .001). (A-E) Data are representative of 2 independent experiments.
Tolerance induction requires IL-10 and is independent of Foxp3+ Treg cells. (A) Mice were treated with hFVIII-alum and anti-CD4 as described in Figure 1A. Different groups were also treated with the indicated mAbs. All mice were exposed to 1 U hFVIII on days 30 and 60. Serum concentrations of hFVIII-specific IgG1 were determined on days 30, 60, and 90 (n = 5; **P < .01; ***P < .001). (B-C) Representative dot-plots (B) and quantification of splenic CD4+CD25+Foxp3+ T cells (C) from animals treated with anti-CD4 mAb or an isotype control on day 90 (n = 5; P = nonsignificant). (D) T/B monoclonal mice (T-Bmc) and T-Bmc Foxp3-deficient mice (T-Bmc FoxP3sf) were immunized on days 1 and 14 with OVA-HA-alum in the presence of nondepleting anti-CD4 or an isotype control. Serum HA-specific IgG1 was determined on day 24 (n = 5; ***P < .001). (E) IL-10−/− and IL-10+/+ mice were treated with hFVIII-alum and anti-CD4 mAbs as described above. The serum concentration of hFVIII-specific IgG1 was quantified on day 90 (n = 6; ***P < .001). (A-E) Data are representative of 2 independent experiments.
These data suggest that Foxp3+ Treg cells may have only a minor role in mediating tolerance in our model. In fact, no differences were found in percentages or absolute numbers of CD4+CD25+Foxp3+ cells in the spleen of animals treated with hFVIII-alum plus anti-CD4 when compared with the positive group (hFVIII-alum plus isotype) 90 days after the induction of tolerance (Figure 2B-C).
As experimental mice have a polyclonal T-cell repertoire, putative changes in the frequency of antigen-specific Treg cells may be beyond the limit of detection. In addition, CD25 depletion with PC61 is known to spare a significant number of Foxp3+ Treg cells.28 We therefore used Foxp3-deficient mice to directly assess if tolerance to a foreign protein could be induced in the absence of Foxp3+ Treg cells. Because Foxp3 deficiency leads to severe autoimmunity, we had to perform this experiment in mice devoid of autoreactive T cells. We used T/B Foxp3sf monoclonal mice (T-Bmc FoxP3sf), characterized by monoclonal populations of T and B lymphocytes specific, respectively, for OVA323-339 and HA.16 As a consequence, we had to use a crosslinked protein of OVA-HA as a model antigen instead of hFVIII. Treatment of T-Bmc mice with OVA-HA–alum and anti-CD4 was equally effective in the presence or absence of functional Foxp3 (Figure 2D).
To further confirm the key role of IL-10 in inducing tolerance to hFVIII, we treated IL10−/− mice with hFVIII-alum plus anti-CD4. We confirmed that it was not possible to prevent the generation of anti-hFVIII IgG1 in IL10−/− mice by treatment with hFVIII-alum plus anti-CD4 (Figure 2E).
Taken together, our data show that induction of tolerance to hFVIII-alum in the presence of anti-CD4 mAb is IL-10 dependent.
Nondepleting anti-CD4 induces long-term prevention from inhibitor formation in hemophilia A mice
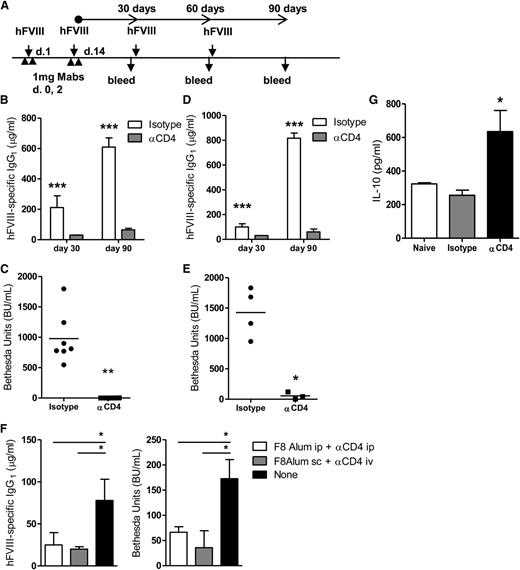
To determine if treatment with anti-CD4 could prevent the formation of FVIII inhibitors in hemophilia, we used mice deficient in FVIII (HemA). C57Bl/6 HemA mice treated with anti-CD4 exhibited a low serum concentration of anti-hFVIII IgG1 for 90 days, even after multiple exposures to hFVIII (Figure 3A-B). In addition, we used the Bethesda assay to measure the titer of FVIII inhibitors at day 90. We found that anti-CD4 treatment successfully prevented the formation of inhibitors in HemA mice (Figure 3C). Similar results were obtained with BALB/c HemA mice (Figure 3D-E). We also found that hFVIII-exposed HemA mice produced high titers of hFVIII-specific immunoglobulin G2a and immunoglobulin G2b, unless treated with an anti-CD4 mAb (supplemental Figure 1). Our findings on the different isotype preferences by the 2 strains of HemA mice are consistent with previous reports.29
Nondepleting anti-CD4 induces long-term protection from inhibitor formation in HemA mice. (A) HemA mice were treated with 1 U hFVIII or HFVIII-alum and 2 × 1 mg nondepleting anti-CD4 (or isotype control) on the indicated days, followed by subsequent administrations of 1 U hFVIII as represented. The presence of serum hFVIII-specific immunoglobulin was investigated at days 30, 60, and 90 following last administration of anti-CD4. (B) Quantification of serum hFVIII-specific IgG1 from C57Bl/6 HemA mice at days 30 and 90, following multiple exposures to hFVIII (n = 8; ***P < .001). (C) Quantification of FVIII inhibitors from the serum of C57Bl/6 mice at day 90 (n = 8; **P < .01). (D) Serum hFVIII-specific IgG1 from BALB/c HemA mice (n = 4; **P < .01). (E) Quantification of hFVIII inhibitors from BALB/c HemA mice (n = 4; *P < .05). (F) C57Bl/6 HemA mice were treated on days 1 and 14 with hFVIII-alum (intraperitoneally or subcutaneously) and anti-CD4 (intraperitoneally or intravenously). A control group remained untreated. Subsequent challenges were done by intravenous administration of hFVIII, in a total of 4 injections, given twice weekly starting on day 21. Quantification of the anti-hFVIII IgG1 levels and BU, measured 1 week after the final exposure to hFVIII (n = 6; *P < .05). (G) Quantification of IL-10 concentration in supernatants of splenocytes from C57Bl/6 HemA mice, collected at day 7 following initial treatment with HFVIII-alum plus anti-CD4. The splenocytes were stimulated with anti-CD3 for 3 days (n = 3; *P < .05). Data are representative of 2 independent experiments. ip, intraperitoneal; iv, intravenous; sc, subcutaneous.
Nondepleting anti-CD4 induces long-term protection from inhibitor formation in HemA mice. (A) HemA mice were treated with 1 U hFVIII or HFVIII-alum and 2 × 1 mg nondepleting anti-CD4 (or isotype control) on the indicated days, followed by subsequent administrations of 1 U hFVIII as represented. The presence of serum hFVIII-specific immunoglobulin was investigated at days 30, 60, and 90 following last administration of anti-CD4. (B) Quantification of serum hFVIII-specific IgG1 from C57Bl/6 HemA mice at days 30 and 90, following multiple exposures to hFVIII (n = 8; ***P < .001). (C) Quantification of FVIII inhibitors from the serum of C57Bl/6 mice at day 90 (n = 8; **P < .01). (D) Serum hFVIII-specific IgG1 from BALB/c HemA mice (n = 4; **P < .01). (E) Quantification of hFVIII inhibitors from BALB/c HemA mice (n = 4; *P < .05). (F) C57Bl/6 HemA mice were treated on days 1 and 14 with hFVIII-alum (intraperitoneally or subcutaneously) and anti-CD4 (intraperitoneally or intravenously). A control group remained untreated. Subsequent challenges were done by intravenous administration of hFVIII, in a total of 4 injections, given twice weekly starting on day 21. Quantification of the anti-hFVIII IgG1 levels and BU, measured 1 week after the final exposure to hFVIII (n = 6; *P < .05). (G) Quantification of IL-10 concentration in supernatants of splenocytes from C57Bl/6 HemA mice, collected at day 7 following initial treatment with HFVIII-alum plus anti-CD4. The splenocytes were stimulated with anti-CD3 for 3 days (n = 3; *P < .05). Data are representative of 2 independent experiments. ip, intraperitoneal; iv, intravenous; sc, subcutaneous.
We confirmed tolerance induction could also be achieved in HemA mice when hFVIII-alum was administered subcutaneously and the anti-CD4 mAb intravenously (Figure 3F).
We also found that splenocytes collected from HemA mice treated with hFVIII-alum plus anti-CD4 when stimulated in vitro in the presence of anti-CD3 were more prone to produce IL-10 than splenocytes from naïve nonmanipulated mice or mice treated with an isotype control (Figure 3G).
Tolerant mice remain immune competent
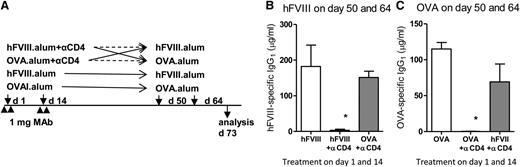
Although the anti-CD4 mAb clone has a nondepleting isotype (ie, it does not directly lead to T-cell lysis), we confirmed that mice treated with anti-CD4 remained immune competent. We used C57Bl/6 mice treated with anti-CD4 as described in Figure 1, but using hFVIII-alum or OVA-alum as the “tolerizing antigen” administered at the time of mAb treatment. We waited 5 weeks to minimize the potential persistence of antigen or anti-CD4. At days 50 and 64, the mice were exposed to the same or to the other antigen (Figure 4A). We found that treatment with anti-CD4 mAb prevented subsequent immunization to the same antigen without suppressing immune responses to the other antigen (Figure 4B-C). In other words, mice tolerized with OVA-alum could respond to subsequent immunization with FVIII-alum, but did not respond to OVA-alum. Conversely, mice tolerized with FVIII-alum did not respond to a later challenge with FVIII-alum, but were fully competent to produce antibodies following immunization with OVA-alum.
Nondepleting anti-CD4–treated mice remain immune competent. (A) C57Bl/6 mice were treated as described previously with anti-CD4, but using FVIII-alum or OVA-alum as the tolerizing antigen administered at the time of mAb treatment. Five weeks following the last mAb infusion, the mice were exposed to the same or to the other antigen. Control groups were not treated with anti-CD4. (B) Some mice were immunized with hFVIII-alum on days 50 and 64, and serum hFVIII-specific IgG1 was quantified at day 73. We found that unlike mice initially treated with OVA-alum plus anti-CD4, mice treated with hFVIII-alum plus anti-CD4 remained unresponsive to the subsequent immunization with hFVIII-alum (n = 4; *P < .05). (C) Other mice were immunized at days 50 and 64 with OVA-alum, and serum OVA-specific IgG1 was quantified on day 73. Animals initially treated with hFVIII-alum plus anti-CD4 remained competent to respond to immunization with OVA-alum, unlike mice initially treated with OVA-alum plus anti-CD4 that remained tolerant to OVA (n = 4; *P < .05). Data are representative of 2 independent experiments.
Nondepleting anti-CD4–treated mice remain immune competent. (A) C57Bl/6 mice were treated as described previously with anti-CD4, but using FVIII-alum or OVA-alum as the tolerizing antigen administered at the time of mAb treatment. Five weeks following the last mAb infusion, the mice were exposed to the same or to the other antigen. Control groups were not treated with anti-CD4. (B) Some mice were immunized with hFVIII-alum on days 50 and 64, and serum hFVIII-specific IgG1 was quantified at day 73. We found that unlike mice initially treated with OVA-alum plus anti-CD4, mice treated with hFVIII-alum plus anti-CD4 remained unresponsive to the subsequent immunization with hFVIII-alum (n = 4; *P < .05). (C) Other mice were immunized at days 50 and 64 with OVA-alum, and serum OVA-specific IgG1 was quantified on day 73. Animals initially treated with hFVIII-alum plus anti-CD4 remained competent to respond to immunization with OVA-alum, unlike mice initially treated with OVA-alum plus anti-CD4 that remained tolerant to OVA (n = 4; *P < .05). Data are representative of 2 independent experiments.
These results show that treatment with anti-CD4 does not induce global immune suppression, but rather antigen-specific unresponsiveness.
Anti-CD4 mAb is effective in inducing tolerance following sensitization
Unlike autoimmunity or allergy, in hemophilia there is an opportunity to induce tolerance in advance of immune sensitization, as the first exposure to the antigens is controlled by the clinician. However, since many hemophilia patients do not develop inhibitors, ITI strategies are often required when the patient is already sensitized. Previous studies suggested that CD4 blockade may be effective in sensitized hosts.30 However, hFVIII-specific immunoglobulin G (IgG) produced at the time of sensitization, given its long half-life, may obscure the eventual effectiveness of tolerance induction at a later time.
To overcome this limitation, and since it is not feasible, in mice, to remove preexisting FVIII-specific IgG by plasmapheresis, we developed an experimental setting to address the role of CD4 blockade in presensitized cells in the absence of preformed FVIII-specific IgG (Figure 5A).
Anti-CD4 mAb is effective in inducing tolerance following sensitization. (A) C57Bl/6 mice were sensitized with hFVIII-alum on days 1 and 14 and sacrificed on day 30. Leukocytes from spleen, pooled lymph nodes, and bone marrow were collected and adoptively transferred intravenously into congenic Rag2−/− or C57Bl/6 recipients. The recipient mice were then treated with hFVIII-alum plus anti-CD4 mAb (or an isotype control) on the day of cell transfer and 14 days later (consistent with protocol represented in Figure 1A), and all groups of mice were subsequently challenged with 3 × 1 U hFVIII on days 10, 12, and 14 following the last administration of anti-CD4. (B) Quantification of serum hFVIII-specific IgG1 in Rag2−/− recipient mice. Animals that received primed T and B cells were competent to produce anti-hFVIII IgG1 (n = 5; ***P < .001), but antibody production was abrogated in mice treated with anti-CD4 (n = 5; ***P < .001). (C) Concentration of serum hFVIII-specific IgG1 in C57Bl/6 mice transferred with primed T and B cells. Animals adoptively transferred with primed cells produced higher levels of hFVIII-specific IgG1 than animals that did not received primed cells (n = 5; ***P < .001). Treatment with anti-CD4 prevented production of hFVIII-specific IgG1 by the primed lymphocytes (n = 5; ***P < .001). Data are representative of 2 independent experiments. (D) C57Bl/6 mice were sensitized with hFVIII on days 1, 5, 10, and 15 and treated with hFVIII-alum and anti-CD4 (intraperitoneally or subcutaneously) on days 22 and 36. Control mice received anti-CD4 without hFVIII. One control group had not been previously exposed to hFVIII. All mice received hFVIII intravenously on days 54, 56, 64, 66, 74, and 76. Anti-hFVIII IgG1 was quantified on the indicated days. Mice treated with hFVIII-alum plus anti-CD4 (regardless of the route of administration) were protected from production of anti-hFVIII IgG1 following subsequent exposure to hFVIII (n = 6; ***P < .001). ip, intraperitoneal; N.D., not detected; sc, subcutaneous.
Anti-CD4 mAb is effective in inducing tolerance following sensitization. (A) C57Bl/6 mice were sensitized with hFVIII-alum on days 1 and 14 and sacrificed on day 30. Leukocytes from spleen, pooled lymph nodes, and bone marrow were collected and adoptively transferred intravenously into congenic Rag2−/− or C57Bl/6 recipients. The recipient mice were then treated with hFVIII-alum plus anti-CD4 mAb (or an isotype control) on the day of cell transfer and 14 days later (consistent with protocol represented in Figure 1A), and all groups of mice were subsequently challenged with 3 × 1 U hFVIII on days 10, 12, and 14 following the last administration of anti-CD4. (B) Quantification of serum hFVIII-specific IgG1 in Rag2−/− recipient mice. Animals that received primed T and B cells were competent to produce anti-hFVIII IgG1 (n = 5; ***P < .001), but antibody production was abrogated in mice treated with anti-CD4 (n = 5; ***P < .001). (C) Concentration of serum hFVIII-specific IgG1 in C57Bl/6 mice transferred with primed T and B cells. Animals adoptively transferred with primed cells produced higher levels of hFVIII-specific IgG1 than animals that did not received primed cells (n = 5; ***P < .001). Treatment with anti-CD4 prevented production of hFVIII-specific IgG1 by the primed lymphocytes (n = 5; ***P < .001). Data are representative of 2 independent experiments. (D) C57Bl/6 mice were sensitized with hFVIII on days 1, 5, 10, and 15 and treated with hFVIII-alum and anti-CD4 (intraperitoneally or subcutaneously) on days 22 and 36. Control mice received anti-CD4 without hFVIII. One control group had not been previously exposed to hFVIII. All mice received hFVIII intravenously on days 54, 56, 64, 66, 74, and 76. Anti-hFVIII IgG1 was quantified on the indicated days. Mice treated with hFVIII-alum plus anti-CD4 (regardless of the route of administration) were protected from production of anti-hFVIII IgG1 following subsequent exposure to hFVIII (n = 6; ***P < .001). ip, intraperitoneal; N.D., not detected; sc, subcutaneous.
In brief, C57Bl/6 mice were immunized with hFVIII-alum on days 1 and 14 and sacrificed on day 30. This immunization protocol leads to high concentration of hFVIII-specific IgG1 (198.0 ± 14.7 μg/mL). We collected and pooled T and B cells from spleen, pooled lymph nodes, and bone marrow. The lymphocytes were then adoptively transferred intravenously into congenic Rag2−/− or C57Bl/6 recipients. The recipient mice were then treated with hFVIII-alum plus anti-CD4 mAb and exposed to additional challenges with hFVIII (Figure 5A).
Using Rag2−/− recipients, we confirmed that the adoptively transferred primed T and B cells were sufficient to produce anti-hFVIII IgG1 (Figure 5B). However, treatment with anti-CD4 was effective in preventing the production of hFVIII-specific IgG1 by those primed cells (Figure 5B).
The adoptive cell transfer into C57Bl/6 mice showed that the adoptively transferred cells were indeed primed. Our data show that recipients of primed cells displayed a rapid and high magnitude secondary-type immune response following exposure to hFVIII when compared with control mice that did not receive primed cells and those that displayed a primary response (Figure 5C). We also observed that when the primed cells were transferred into C57Bl/6 mice, treatment with anti-CD4 mAb prevented production of anti-hFVIII IgG1 (Figure 5C).
We then investigated whether hFVIII-alum plus anti-CD4 could protect mice sensitized to hFVIII from further production of FVIII-specific antibodies. We found that animals exposed to hFVIII, when subsequently treated with hFVIII-alum (intraperitoneally or subcutaneously) plus anti-CD4, were protected from an increase in serum levels of FVIII-specific IgG1, in spite of repeated administrations of hFVIII (Figure 5D). The effect was not due to nonspecific effects of anti-CD4, as mice treated with anti-CD4 in the absence of hFVIII responded to subsequent exposure to hFVIII with production of hFVIII-specific IgG1.
These results demonstrate that anti-CD4 mAb treatment is effective in controlling the response to hFVIII in presensitized animals.
Discussion
Our results show that an adjuvant (alum) can enhance the ability of nondepleting anti-CD4 mAb to achieve tolerance to hFVIII. These results can appear counterintuitive, as adjuvants such as alum are usually used to boost immunity, leading to the production of protective antibodies. It is likely that when tolerance is based on CD4 blockade, it will be necessary that the majority of antigen-specific T cells will be exposed to the antigen during the time when the CD4-blocking mAb is present and with optimal costimulation. In this regard, our results on the antigen specificity of tolerance (Figure 4) also confirm that tolerance is imposed on the lymphocytes that are engaged in antigen recognition at the time of CD4 blockade. Our data show that exposure to hFVIII-alum plus anti-CD4 can prevent subsequent immune responses targeting hFVIII while allowing successful immunization to an unrelated antigen (OVA). Similar results were obtained in the converse experiment: when tolerance is induced with OVA-alum plus anti-CD4, the animals can produce antibodies targeting hFVIII, following exposure to the clotting factor. We can conclude that the initial exposure to hFVIII-alum is therefore critical to control the FVIII-specific T-cell clones, but does not affect T-cell clones with other specificities.
Given previous reports showing a clear role of Foxp3+ Treg cells in transplantation tolerance induced with anti-CD4,9,26,31 it was surprising to find that tolerance induction to proteins in alum could be achieved in the absence of Foxp3 (Figure 2). We have confirmed that tolerance induction to skin grafts, induced following anti-CD4 mAb treatment, is indeed dependent on Foxp3+ Treg cells (data not shown). In the same fashion, several animal models of autoimmunity can be successfully treated with anti-CD4, by a mechanism that appears to be Foxp3 dependent.32 Inhibition of mTOR has been shown to facilitate the induction of Foxp3+ Treg cells and a strategy to improve the efficacy of induction of transplantation tolerance.33 It was also reported that treatment with rapamycin (an mTOR inhibitor) can lead to tolerance to FVIII.34
Our data show that the systemic distribution of a protein in alum appears to take advantage of an IL-10–dependent and Treg-independent mechanism. This observation may explain the apparently contradictory results from transplantation studies using anti-CD4 for tolerance induction. Although tolerance appears to be IL-10 independent and Treg dependent in studies where a skin graft alone is used,9,35 studies where donor-specific transfusion and a skin graft are used appear to rely on both Treg cells and IL-10,24,27 suggesting that systemic distribution of the antigen may recruit a IL-10–mediated tolerogenic mechanism. It remains to be established the reason for the absolute requirement of Foxp3+ Treg cells in transplantation tolerance,31 while the same clone of nondepleting anti-CD4 mAb leads to Foxp3-independent immune tolerance in hemophilia.
By neutralizing IL-10R in vivo during the first 2 weeks of anti-CD4 treatment, we could infer the participation of IL-10 in the mechanism leading to tolerance, but we could also establish the importance of that initial period for tolerance induction (Figure 2). This observation is consistent with the need to impose tolerance in antigen-specific cells during a window of time, after which the immune system regains the ability to respond to foreign antigens, as discussed above. In addition, by showing that repeated administrations of hFVIII (without alum) during those initial 2 weeks did not lead to long-term tolerance, as we observed when hFVIII-alum was used (Figure 1C), we were able to exclude the possibility that tolerance facilitation by alum could be simply due to the depot effect leading to greater half-life of the antigen.
A usual concern in transplantation tolerance experiments is the influence of the genetic background in the results.36 Some strains, namely C3H and CBA/Ca, are known to be more permissive to the induction of transplantation tolerance than other strains, such as C57Bl/6 and BALB/c. In order to evaluate whether tolerance to hFVIII was strain specific, we confirmed that long-term tolerance to hFVIII could be achieved in C57Bl/6 and BALB/c mice and in mice from those 2 strains deficient in FVIII (HemA mice). When we used hemophilic mice, we could directly document the physiological impact of FVIII inhibitors, not only by their quantification using the Bethesda assay, but also by our subjective assessment of bleeding from those mice. Treatment with hFVIII-alum plus anti-CD4 led to long-term protection from the generation of inhibitors in the 2 strains of hemophilic mice.
Several other mAbs targeting T-cell coreceptors (namely anti-CD3) and costimulatory molecules have been shown to be effective in inducing immune tolerance in transplantation, autoimmunity, and allergic diseases.8,37 Recently, it was reported that antibodies targeting CD3 can induce long-term tolerance to FVIII in hemophilic mice.38 In addition, mAbs targeting ICOS are effective in inducing long-term tolerance to hFVIII in mice following gene therapy.14 Targeting B cells with an anti-CD20 IgG1 is another approach that could lead to tolerance to FVIII.39 Concerning nondepleting anti-CD4, there is, however, one special case of successful induction of long-term tolerance to foreign proteins in the absence of adjuvant: it is when the foreign protein is an immunoglobulin. In fact, in some of the earliest demonstrations that nondepleting anti-CD4 can induce tolerance to foreign antigens, immunoglobulins from different animal species were used as the tolerizing antigen.40,41 It is likely that the special tolerogenicity of foreign immunoglobulins compared with FVIII represents the fact that antigen-presenting cells can capture foreign immunoglobulins with specific Fc receptors for efficient presentation.
Given the promising preclinical results, anti-CD4 mAb was among the very first mAb to enter clinical trials.42,43 The outcome of those early clinical trials was disappointing, which led to the search for alternative tolerance-inducing strategies. In retrospect, however, it is possible that the low efficacy was due to immaturity of the field. In fact, clinical trials in patients with rheumatoid arthritis and multiple sclerosis were not performed with humanized mAb, and the low efficacy correlated well with the generation of immunoglobulins targeting the therapeutic mAb.44 In addition, in a well-known clinical trial in asthma, a different anti-CD4 mAb was used (keliximab) that caused significant T-cell depletion.45 More recently, preclinical studies with a humanized nondepleting anti-CD4 have shown it could induce tolerance in nonhuman primates to foreign immunoglobulins.46 It is possible that the use of alum as an adjuvant, as we now describe, will allow tolerance induction to not only hFVIII but also other therapeutic recombinant proteins, namely in enzyme replacement therapy. A potential limitation for the clinical application of this approach is the perceived risk of inducing further production of FVIII inhibitors with the use of an adjuvant (alum).
It is well established that the primed immune system is more resistant to tolerance-inducing strategies. Nevertheless, most clinical situations would require tolerance induction in an immunized individual. In hemophilia, FVIII inhibitors are only produced in a proportion of patients. Therefore, tolerance induction should only be initiated in the patients who would require this intervention, and those patients can only be identified after the onset of inhibitors. However, the study of tolerance-inducing strategies in mice previously immunized to hFVIII has been difficult due to the long half-life of hFVIII-specific IgG, the experimental readout. As a consequence, most studies in mice are restricted to the ability to induce tolerance at the time of the initial exposure to hFVIII, an event that is controlled by the clinician. We developed an experimental system to study the effect of hFVIII-alum plus anti-CD4 on the primed immune system (Figure 5). Instead of removing preexistent hFVIII-specific IgG by plasmapheresis—something that we could not do in mice—we transferred primed immune cells to a new host. In this way, it became possible to confirm that our protocol could prevent de novo production of hFVIII-specific antibodies by the primed cells. Such an adoptive cell-transfer system is unlikely to reproduce in its entirety the characteristics of a primed immune system. As a consequence, we confirmed that mice exposed to hFVIII (bearing hFVIII-specific IgG1), when treated with hFVIII-alum plus anti-CD4, were protected from further production of hFVIII-specific antibodies following subsequent hFVIII infusions. These findings are consistent with our previous report that anti-CD4 is able to prevent allergic airways disease in mice presensitized to an allergen (house dust mite).30 Furthermore, a report has shown that mAbs targeting ICOS and CD154 can prevent memory plasma cells from responding to FVIII,47 and treatment of FVIII-primed mice with B cells expressing the immunodominant FVIII C2 and A2 domains was shown to lead to humoral tolerance.48
Taken together, our results show that alum, in spite of its common use to boost immune responses, can be effective in enhancing the ability of nondepleting anti-CD4 mAb to induce long-term and antigen-specific tolerance to hFVIII in mice. The tolerogenic mechanism relies on IL-10 and is independent of Foxp3+ Treg cells. We believe that the use of alum, or other adjuvants, may constitute a strategy that could be applied to different immunogenic therapeutic proteins and other tolerance-inducing mAbs. In this case, our findings may be of interest beyond the field of hemophilia, namely in regard to enzyme replacement therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jocelyne Demengeot, Ruy M. Ribeiro, and Bruno Silva-Santos for helpful advice, Joana Duarte for assistance in some experiments, David Lillicrap for advice and for providing HemA mice, and Herman Waldmann for providing several hybridomas used in our study.
This work was funded by Fundacao para a Ciência e Tecnologia Portugal (PTDC/SAU-OSM/108267/2008 and PTDC/SAU-TOX/114424/2009), the Bayer Global Hemophilia Award (V.G.O.), and the CSL-Behring Professor Heimburger Award (L.G.).
Authorship
Contribution: V.G.O. and A.A.-D. designed and performed research, analyzed data, and wrote the paper; M.A.C.d.L. and J.J.L. contributed vital new reagents and analyzed data; L.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.A.C.d.L. is Singapore Immunology Network, Agency for Science, Technology and Research, 138648, Singapore.
Correspondence: Vanessa G. Oliveira, Instituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisbon, Portugal; e-mail: vgoliveira@fm.ul.pt; and Luis Graca, Instituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisbon, Portugal; e-mail: lgraca@fm.ul.pt.
References
Author notes
V.G.O. and A.A.-D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal