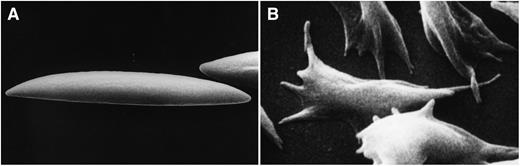
(A) Membrane deformation induced by fluid shear stress acting on normal human discoid red cells and (B) membrane deformation induced by polymerization of hemoglobin upon deoxygenation of human sickle red cells. Do these types of membrane deformation regulate PIEZO1 function?
(A) Membrane deformation induced by fluid shear stress acting on normal human discoid red cells and (B) membrane deformation induced by polymerization of hemoglobin upon deoxygenation of human sickle red cells. Do these types of membrane deformation regulate PIEZO1 function?
The shrinking cell has been an object of curiosity and some bafflement to physiologists and hematologists since it was first seen some 40 years ago. We know that regulation of its volume within narrow limits through a tightly controlled intracellular cation concentration is critical for optimal functioning and survival of the red cell. An autosomal dominant hemolytic anemia characterized by primary red cell dehydration due to decreased cation content was first described by Miller and colleagues in 19712 and is currently designated as hereditary xerocytosis (HX) or DHSt. These patients typically exhibit mild to moderately compensated hemolytic anemia and the red cells are characterized by increased mean corpuscular hemoglobin concentration and decreased osmotic fragility, both reflecting cellular dehydration. In addition to anemia, a subset of the patients exhibit pre- and/or perinatal edema which recedes spontaneously.3,4
The molecular basis of the disorder, which has been under intense scrutiny for decades, was recently resolved thanks to the identification of mutations in the gene encoding PIEZO1. These came to light in 2 large kindreds studied by Zarychanski and colleagues last year5 and in 7 additional families described in the present study.1 PIEZO1 was identified as a protein involved in mechanosensation and stretch-activated cation channel regulation in 2010,6 and it adds to the impact of that work that, 2 years later, a red cell disorder has been identified as the first human disease stemming from mutations in this gene.
Although the identification of mutations in PIEZO1 leading to red cell dehydration in HX by 2 independent groups is a cause for satisfaction and opens new avenues of research toward a further understanding of red cell volume regulation, a number of questions remain unanswered. Whereas the finding that PIEZO1 protein is expressed in erythroblasts and is present in the membrane of the mature cell could account for dehydration of reticulocytes, as well as of red cells in HX, the mechanism of cell dehydration of erythroid cells remains to be defined. Furthermore, because the newly recognized function of PIEZO1 as a stretch-activated cation channel can be rationalized as an essential element in red cell volume regulation during repeated cycles of membrane deformation during passage through the microvasculature (panel A), it is not clear how its function is regulated in erythroblasts in the relatively static bone marrow environment.
Another unresolved question is how the various identified mutations in PIEZO1 account for the large phenotypic variability in the clinical expression between patients. Similarly, while the documented expression of PIEZO1 in fetal lymphatic vessel endothelium suggests a potential causative role for the protein in the pathogenesis of perinatal effusions, it not clear why only a subset of HX patients with mutations in PIEZO1 exhibits this clinical syndrome.
What then are the implications of these findings? One is that PIEZO1, as the newest member of transport proteins responsible for regulating cation content of red cells, will advance our mechanistic understanding of disordered volume regulation, not only in HX but also in a number of other red cell disorders, including sickle cells.7,8 It will indeed be exciting if PIEZO1 can be shown to play a key role in the well-documented deoxygenation-induced increase in cation permeability of sickle red cells; for this phenomenon is responsible for pathogenic cell dehydration, contingent on membrane deformation (panel B).9 The study by Adolfo and his colleagues1 represents the first step in what should prove a long and productive journey toward a comprehensive understanding of red cell hydration pathways in health and disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.