Key Points
Metastatic tumor cell attachment induces endothelial VCAM-1 and VAP-1.
VCAM-1 and VAP-1 promote metastatic tumor cell survival by recruiting myeloid cells, pointing to VAP-1 as a therapeutic target.
Abstract
Pulmonary metastasis is a frequent cause of poor outcome in cancer patients. The formation of pulmonary metastasis is greatly facilitated by recruitment of myeloid cells, which are crucial for tumor cell survival and extravasation. During inflammation, homing of myeloid cells is mediated by endothelial activation, raising the question of a potential role for endothelial activation in myeloid cell recruitment during pulmonary metastasis. Here, we show that metastatic tumor cell attachment causes the induction of the endothelial activation markers vascular cell adhesion molecule-1 (VCAM-1) and vascular adhesion protein-1 (VAP-1). Induction of VCAM-1 is dependent on tumor cell-clot formation, decreasing upon induction of tissue factor pathway inhibitor or treatment with hirudin. Furthermore, inhibition of endothelial activation with a VCAM-1 blocking antibody or a VAP-1 small molecule inhibitor leads to reduced myeloid cell recruitment and diminished tumor cell survival and metastasis without affecting tumor cell adhesion. Simultaneous inhibition of VCAM-1 and VAP-1 does not result in further reduction in myeloid cell recruitment and tumor cell survival, suggesting that both act through closely related mechanisms. These results establish VCAM-1 and VAP-1 as mediators of myeloid cell recruitment in metastasis and identify VAP-1 as a potential target for therapeutic intervention to combat early metastasis.
Introduction
Metastasis can be greatly facilitated by the recruitment of myeloid cells. Because leukocyte homing during inflammation is mediated by endothelial activation, we examined the role of endothelial activation in myeloid cell recruitment during early pulmonary metastasis. Inflammation results in luminal expression of a variety of molecules that mediate adhesion between the blood-borne inflammatory cells and the endothelium.1,2 One class of these molecules is the selectins, which are lectins that bind oligosaccharides on leukocytes with relatively low affinity. These interactions reduce the velocity of the leukocytes in the bloodstream, making them appear to roll on the surface of the blood vessel. Tighter adhesion and arrest are then mediated by integrin binding between the leukocyte and the endothelial activation markers markers vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule (ICAM)-1 and -2, which are transcriptionally induced and expressed on the endothelial luminal surface.
In addition to these molecules, vascular adhesion protein-1 (VAP-1), an ectoenzyme that is stored in cytoplasmic vesicles and upon inflammatory stimulation is relocated to the luminal surface of endothelial cells, has a dual action in endothelial adhesion.3,4 As an adhesion molecule, it binds different leukocyte ligands, including Siglec-9 and Siglec-10, which are present on granulocytes monocytes and B cells, respectively. As an enzyme, it converts primary amines into aldehyde through its semicarbazide-sensitive amine oxidase (SSAO) activity, releasing hydrogen peroxide and ammonium. VAP-1 enzymatic activity enhances cellular binding, rolling, and firm adhesion during inflammation, playing mostly a supplemental role.5-7 VAP-1−deficient mice have impaired leukocyte trafficking into mesenteric lymph nodes, spleen, peritoneum, and joints during inflammation8 ; modestly diminished T- and B-cell responses; and a mildly diminished response to bacterial (Staphylococcus aureus) or viral (Coxsackie B4) infections. However, inhibition of VAP-1 activity in wild-type adult mice with antibodies or pharmaceutical agents did not alter the response to these infections.9 Leukocyte homing to liver sinusoids, especially of CD16+ monocytes, might be more dependent upon VAP-1 than homing at other sites.10-12 M2 proangiogenic monocytes may be especially reliant on VAP-1 for homing.13 The VAP-1 gene is amplified in gastric cancer,14 and VAP-1 is expressed in the blood vessels of human tumors of the head and neck, liver, and melanoma.15,16 Interestingly, inhibition of VAP-1 catalytic activity (but not VAP-1 blockade with antibodies) results in reduction in myeloid cell infiltration (CD11b+, Gr-1+), a modest reduction in tumor growth, and impaired neoangiogenesis.17,18
Lung colonization by metastatic tumor cells results in a rapid influx of myeloid cells that are essential for survival and extravasation of the metastatic tumor cells.19-22 Many cancer cells express procoagulant molecules, such as tissue factor (TF), resulting in clot formation by arrested tumor cells. These clots trigger the recruitment of CD11b+, CD11c−, and Gr1− myeloid cells.19 At later times, after tumor cell arrest, CCL2/MCP-1 released by the tumor cells also facilitates the recruitment of myeloid cells.22
The similarity of the process of tumor cell arrest in a vascular bed during metastasis to leukocyte homing has raised the possibility that the metastatic process uses the same mechanisms. Stimulation of endothelial activation by inflammatory cytokines, including tumor necrosis factor-α, interleukin-1, and CXCL12, indeed leads to enhanced metastatic colonization mediated by VCAM-1, especially by tumor cells expressing the α4β1 integrin VLA-4, the ligand of VCAM-1.23-26 However, inhibiting VCAM-1 before the introduction of tumor cells does not block the basal level of lung colonization (Okahara et al24 and Špela Ferjančič, Ana M. Gil-Bernabe, Sally A. Hill, Phiip D. Allen and Ruth J. Muschel, 2009-2012, unpublished data), suggesting that the initial metastatic cell attachment can occur independently of VCAM-1. VCAM-1 aberrantly expressed by breast cancer cells interacts with macrophages via α4 integrins, stimulating lung colonization.27 Perhaps this is a redundant mechanism for the recruitment of macrophages by other means. Interaction of myeloid cells through tumor cell VCAM-1 can ablate tumor cell dormancy in the bone marrow, enhancing bone metastasis.28 Thus, VCAM-1 expression appears to augment metastasis especially by tumor cells that express the α4β1 integrin but is not required for tumor cell adhesion.
Therefore, perhaps instead of endothelial activation driving tumor cell arrest, tumor cell arrest can drive endothelial activation. Supporting this hypothesis, the induction of VCAM-1 and ICAM-1 soon after the attachment of tumor cells in the liver has been reported.29 Similarly, in the brain, VCAM-1 was not initially evident but was detected soon after adhesion and has been proposed as a surrogate marker for the detection of brain metastasis.30 In this study, we show that endothelial activation through both VCAM-1 and VAP-1 can contribute to the recruitment of myeloid cells that are essential for tumor cell survival. Inhibition of VCAM-1 or VAP-1 diminished lung colony formation by decreasing the recruitment of myeloid cells.
Methods
Cell culture and cell staining
4T1-green fluorescent protein (GFP) murine breast cancer cells, 1205Lu-GFP human melanoma cells,31 the highly metastatic cell line, Met-1, derived from a PyMT mouse mammary tumor,20 and the murine B16F10, B16F10-TF pathway inhibitor (TFPI) and B16F10-vector (pcDNA3.1/Zeo) melanoma cell lines32 were cultured as detailed in supplemental Methods (see the Blood Web site). Cells were stained with CMFDA or CMRA (12.5µM; Molecular Probes), following the manufacturer’s protocol.
Animals and drug treatments
All animal procedures were conducted in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and following local ethics review regulated by the Home Office U.K. and the University of Oxford Clinical Medicine Ethical Review Committee. Severe combined immunodeficient (SCID; CB17/Icr-Prkdcscid/IcrCrl), BALB/c, and C57BL/6J mice were purchased from Charles River Laboratories. Cx3cr1gfp/+ mice (B6.129P-Cx3cr1tm1Litt/J) were obtained from The Jackson Laboratory.33 Csf1r-GFP FVB [FVB.(tg(Csf1r-EGFP)1Jwp)] and B6.Mac1-knockout (KO) mice were gifts from Professor Jeffrey W. Pollard, Albert Einstein College of Medicine, New York,20 and Siamon Gordon, University of Oxford, United Kingdom,34 respectively.
Recombinant hirudin (Refludan; Pharmion) was administered intraperitoneally at 20 mg/kg,35 5 minutes before and 4 hours after the intravenous injection of tumor cells. Lipopolysaccharides (LPS; Sigma-Aldrich) were administered intraperitoneally at 5 mg/kg. VCAM-1 blocking antibody (clone M/K-2; Millipore) and its corresponding IgG1-κ isotype control (Gene Tex Inc) were administered intravenously at 1.5 mg/kg 4 hours before the intravenous injection of tumor cells. VAP-1 inhibitor, PRX.A (Proximagen) was administered intraperitoneally at 6 mg/kg 1 hour before the intravenous injection of tumor cells, or as indicated otherwise. PRX.A causes 50% inhibition of mouse SSAO at 0.7 nM and is selective over 75 other receptors and ion channels in vitro, except for the dopamine transporter and the batrachotoxin voltage-sensitive Na channel site (<50% inhibition of both is observed at 1 µM). The free plasma concentrations achieved would be expected to result in >90% inhibition of the enzyme for at least 5 hours, without exceeding 100 nM, to ensure selectivity.
Immunohistochemistry
Murine tissue sections (described in the supplemental Methods) were stained with the following: rat anti-E-selectin (ABR Affinity BioReagents), rat anti-VCAM-1 (R&D Systems), rat anti-CD11b, rat anti-VAP-1 (Abcam), and rat anti-αIIb (Santa Cruz Biotechnologies). The TSA biotin amplification system (PerkinElmer) was performed with the use of biotinylated secondary antibodies (goat anti-rat IgG; Zymed Laboratories) and streptavidin-conjugated fluorophores (streptavidin-Alexa Fluor 488 and streptavidin-Alexa Fluor 633; Invitrogen).
Microscopy and image analysis
Images were acquired via an inverted epifluorescence microscope (DM IRBE; Leica Microsystems) and a digital camera (Orca; Hamamatsu Photonics), and analyzed with Simple PCI 6.5 (Hamamatsu Photonics) and ImageJ 1.46 (http://rsb.info.nih.gov/ij/) software. Confocal microscopy was performed as described in supplemental Methods. The percentage of positive area was analyzed with ImageJ software (http://rsb.info.nih.gov/ij/) after we set an appropriate threshold. Tumor cells were considered to be associated with an endothelial cell activation antigen when expression of this antigen was observed at a distance of <80 µm from a tumor cell, the critical oxygen diffusion distance in respiring tissue.36 Recruitment of myeloid cells was considered positive when 5 or more cells were clustered around the tumor cell.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5.02, as described in the supplemental Methods. The particular test performed in each experiment is indicated in the corresponding figure legend. Differences were considered significant with P < .05. Data represent mean + or ± SD, unless specified otherwise.
Results
VCAM-1 and VAP-1 are induced in experimental lung metastasis
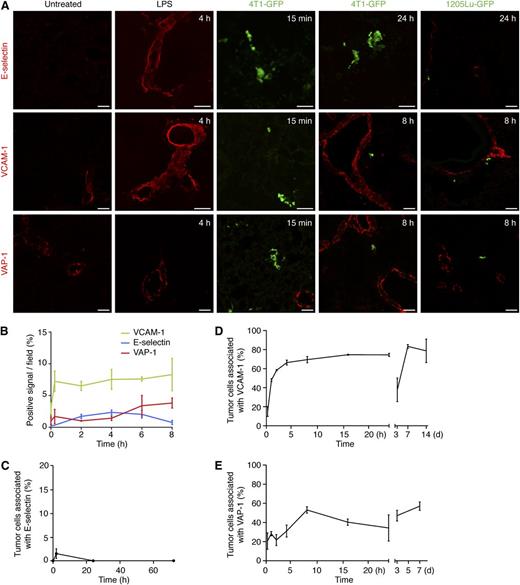
E-selectin was not detected in the unstimulated lung endothelium, whereas VCAM-1 and VAP-1 were only expressed at low levels (Figure 1A, untreated, and 1B, time zero). LPS were injected intraperitoneally as a positive control. E-selectin and VCAM-1 were induced after LPS treatment (Figure 1A, LPS, and 1B; supplemental Figure 1). VAP-1 expression was further up-regulated after treatment with LPS (Figure 1A-B and supplemental Figure 1). Although P-selectin and ICAM-1 also were up-regulated after inflammation, the high basal expression levels of ICAM-1 in the resting lung and the presence of P-selectin in platelets made them unsuitable for further evaluation in this study (data not shown).
Endothelial activation in response to LPS treatment and to tumor cell challenge. BALB/c mice were treated with LPS or injected with 5 × 105 4T1-GFP tumor cells (green) intravenously. SCID mice were injected with 5 × 105 1205Lu-GFP tumor cells (green) intravenously. Lungs were harvested at the indicated times and analyzed by immunohistochemistry for the presence of different antigens of endothelial activation. (A) Representative images, acquired with a confocal microscope, of stainings for E-selectin, VCAM-1, and VAP-1 are shown (Alexa Fluor 633, red). (B) Dynamics of endothelial activation upon LPS treatment. Time zero represents untreated mice and indicates the basal expression levels of the adhesion molecules. Data represent mean ± SD, n = 3 mice. Representative images are shown in supplemental Figure 1. E-selectin (C), VCAM-1 (D), and VAP-1 (E) induction upon challenge with 4T1-GFP tumor cells was evaluated as described in the “Microscopy and image analysis” subsection of “Methods”. In (C-E) n ≥3 mice and data represent (C) mean ± SD or (D-E) mean ± SEM. In (C) values corresponding to the time points 15 minutes, 24 hours, and 72 hours are zero. Scale bars represent 50 µm.
Endothelial activation in response to LPS treatment and to tumor cell challenge. BALB/c mice were treated with LPS or injected with 5 × 105 4T1-GFP tumor cells (green) intravenously. SCID mice were injected with 5 × 105 1205Lu-GFP tumor cells (green) intravenously. Lungs were harvested at the indicated times and analyzed by immunohistochemistry for the presence of different antigens of endothelial activation. (A) Representative images, acquired with a confocal microscope, of stainings for E-selectin, VCAM-1, and VAP-1 are shown (Alexa Fluor 633, red). (B) Dynamics of endothelial activation upon LPS treatment. Time zero represents untreated mice and indicates the basal expression levels of the adhesion molecules. Data represent mean ± SD, n = 3 mice. Representative images are shown in supplemental Figure 1. E-selectin (C), VCAM-1 (D), and VAP-1 (E) induction upon challenge with 4T1-GFP tumor cells was evaluated as described in the “Microscopy and image analysis” subsection of “Methods”. In (C-E) n ≥3 mice and data represent (C) mean ± SD or (D-E) mean ± SEM. In (C) values corresponding to the time points 15 minutes, 24 hours, and 72 hours are zero. Scale bars represent 50 µm.
Next, we investigated endothelial activation after tumor cell arrest in the pulmonary bed in both immunocompromised (SCID) and immunocompetent (BALB/c, syngeneic for 4T1 tumor cells) hosts. E-selectin induction was variable, depending on the model, that is, not induced after 4T1 cell introduction, slightly induced by 1205Lu cells (Figure 1A, top, and 1C), and strongly, but transiently, induced after introduction of MDA-MB-231 cells (supplemental Figure 2A). VCAM-1 was induced in the endothelium in proximity to the tumor cells within 2h of their intravenous introduction (Figure 1A, middle, and 1D; supplemental Figure 2A), and localized with the endothelial marker von Willebrand factor (supplemental Figure 2B). We did not detect VCAM-1 expression on the tumor cells. There was a reproducible, but modest, regression in the extent of VCAM-1 staining between 1 and 3 days after tumor cell introduction. VCAM-1 staining persisted and increased afterward (Figure 1D; supplemental Figure 2A). Although some increase in VCAM-1 staining could be attributed to angiogenesis, the bulk of the staining appeared on vessels adjacent to, but not part of, the colonies (supplemental Figure 2C). VCAM-1 expression in the vicinity of adherent tumor cells was also found in other models of metastasis, including spontaneous metastasis to the lung of mice bearing subcutaneous 4T1 tumors, pulmonary metastasis of LLC cells, and liver metastasis of MC38 cells (supplemental Figure 2D). VAP-1 staining in the vicinity of adherent 4T1 and 1205Lu tumor cells increased over basal levels with time, as did the percentage of tumor cells associated with VAP-1 expression (Figure 1A, bottom, and 1E). Thus, the attachment of tumor cells consistently induced local endothelial expression of VCAM-1 and VAP-1.
VCAM-1 expression is associated with monocyte/macrophage recruitment by the tumor cells
We have previously shown that tumor cells induce clot formation that in turn induces myeloid cell recruitment.19,31 B16F10 and Met-1 tumor cells were respectively introduced into Cx3cr1gfp/+, in a C57BL/6 background, and Csf1r-GFP, in an FVB background, mice, in which populations of myeloid cells are fluorescently labeled.20,33 VCAM-1 expression was induced in the vicinity of the tumor cell-clot-monocyte/macrophage aggregate (Figure 2; supplemental Figure 3A). Clot formation around the tumor cell and recruitment of CD11b+ cells also were observed in nontransgenic mice (supplemental Figure 3B). Both B16F10 and 4T1 cells expressed TF, leading to tumor cell clot formation and monocyte/macrophage recruitment; in contrast to 1205Lu cells, which did not express TF (supplemental Figure 3C) and failed to induce extensive clot formation or monocyte/macrophage recruitment (supplemental Figure 3B). 4T1 tumor cells attracted a similar population of myeloid cells compared with B16F10 cells (CD11b+, CD11c−, F4/80+, CD68+, CX3CR1+, CD45+, Gr-1−, CD3e−).19,20 4T1 cells are known to attract high numbers of Gr-1+ CD11b+ myeloid-derived suppressor cells.37
VCAM-1 expression is associated with myeloid cell recruitment.Cx3cr1gfp/+ (left panels; green, myeloid cells) or Csf1r-GFP mice (right panels; green, myeloid cells) were injected intravenously with 5 × 105 CMRA-stained B16F10 or -Met-1 cells, respectively (magenta). Eight hours later, lungs were harvested and assessed by immunohistochemistry for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope from 3 independent experiments performed, are shown. Scale bars represent 50 µm.
VCAM-1 expression is associated with myeloid cell recruitment.Cx3cr1gfp/+ (left panels; green, myeloid cells) or Csf1r-GFP mice (right panels; green, myeloid cells) were injected intravenously with 5 × 105 CMRA-stained B16F10 or -Met-1 cells, respectively (magenta). Eight hours later, lungs were harvested and assessed by immunohistochemistry for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope from 3 independent experiments performed, are shown. Scale bars represent 50 µm.
Clot formation contributes to endothelial cell activation in pulmonary metastasis
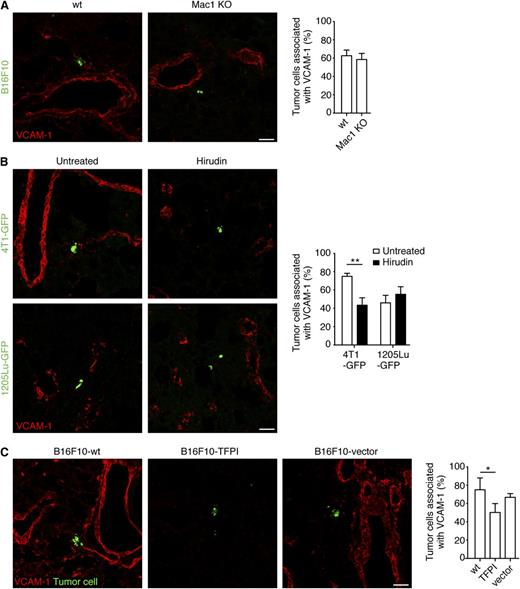
To determine the relationship between monocyte/macrophage recruitment and endothelial activation, we analyzed the induction of VCAM-1 in a mouse model with impaired function of monocytes/macrophages, the Mac1 KO mouse. This mouse lacks the αM subunit (CD11b) of the αMβ2 (CD11b/CD18) heterodimeric integrin. As previously reported, the recruitment of myeloid cells (CD45+ and F4/80+ cells) to the tumor cells was decreased in the Mac1 KO mouse, although clot formation was not affected.19 Eight hours after the introduction of B16F10 cells, VCAM-1 was expressed in the vicinity of tumor cells despite diminished CD11b expression (Figure 3A). These results indicate that monocytes/macrophages are not responsible for endothelial activation in early metastasis.
Clot formation contributes to endothelial activation in pulmonary metastasis. (A) C57BL/6-wt or Mac1 KO mice were intravenously injected with 5 × 105 CMFDA-stained B16F10 tumor cells (green). Lungs were harvested 8 hours later and assessed for VCAM-1 expression (red, Alexa Fluor 633; imaged with a confocal microscope). Percentage of tumor cells associated with VCAM-1 expression is shown; n = 3 mice (Mann-Whitney). (B) BALB/c or SCID mice were treated with hirudin and intravenously injected 5 × 105 4T1-GFP or 1205Lu-GFP tumor cells, respectively. Eight hours after the introduction of tumor cells, lungs were harvested and assessed for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope, are shown. Percentage of tumor cells associated with VCAM-1 expression was analyzed; n ≥ 4 mice (Mann-Whitney for each cell line). (C) C57BL/6 mice were intravenously injected 5 × 105 CMFDA-stained-B16F10-wt, -B16F10-TFPI, or -B16F10-vector tumor cells (green). Lungs were harvested 8 hours after and assessed for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope, are shown. Percentage of tumor cells associated with VCAM-1 expression was analyzed; n = 3 mice (one-way analysis of variance and the Tukey test). In (A-C), data represent mean + SD and *P < .05; **P < .01. Scale bars represent 50 µm.
Clot formation contributes to endothelial activation in pulmonary metastasis. (A) C57BL/6-wt or Mac1 KO mice were intravenously injected with 5 × 105 CMFDA-stained B16F10 tumor cells (green). Lungs were harvested 8 hours later and assessed for VCAM-1 expression (red, Alexa Fluor 633; imaged with a confocal microscope). Percentage of tumor cells associated with VCAM-1 expression is shown; n = 3 mice (Mann-Whitney). (B) BALB/c or SCID mice were treated with hirudin and intravenously injected 5 × 105 4T1-GFP or 1205Lu-GFP tumor cells, respectively. Eight hours after the introduction of tumor cells, lungs were harvested and assessed for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope, are shown. Percentage of tumor cells associated with VCAM-1 expression was analyzed; n ≥ 4 mice (Mann-Whitney for each cell line). (C) C57BL/6 mice were intravenously injected 5 × 105 CMFDA-stained-B16F10-wt, -B16F10-TFPI, or -B16F10-vector tumor cells (green). Lungs were harvested 8 hours after and assessed for VCAM-1 expression (red, Alexa Fluor 633). Representative images, acquired with a confocal microscope, are shown. Percentage of tumor cells associated with VCAM-1 expression was analyzed; n = 3 mice (one-way analysis of variance and the Tukey test). In (A-C), data represent mean + SD and *P < .05; **P < .01. Scale bars represent 50 µm.
Inhibition of coagulation reduced endothelial activation induced by tumor cells. Hirudin, a direct inhibitor of thrombin, decreased the formation of clots by 4T1 tumor cells (supplemental Figure 4A), and the recruitment of CD11b+ (supplemental Figure 4B), Gr-1+, and CD45+ (supplemental Figure 4C) cells. Hirudin reduced VCAM-1 induction in this model (Figure 3B). In contrast, mice injected with 1205Lu cells had no alteration in the already-low levels of monocyte/macrophage recruitment or VCAM-1 induction after treatment with hirudin (Figure 3B; supplemental Figure 4B). Similarly, B16F10 tumor cells expressing the endogenous inhibitor of TF, B16F10-TFPI cells, had reduced clot formation, recruitment of monocyte/macrophages and VCAM-1 expression in the vicinity of the tumor cells (supplemental Figure 5; Figure 3C), confirming that coagulation induced by tumor cells is a contributor to VCAM-1 induction. Cytokine expression profiles of 3 of the cell lines (4T1-GFP, 1205Lu-GFP, and MDA-MB-231-GFP) were obtained (supplemental Figure 6), but no correlation with endothelial cell induction was apparent. In summary, abrogation of coagulation induced by tumor cells reduced, but did not eliminate, endothelial activation.
Inhibition of endothelial activation results in decreased recruitment of monocytes/macrophages and reduced metastasis
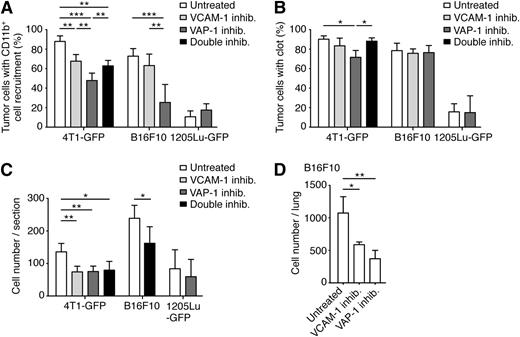
Treatment with VCAM-1 blocking antibody reduced the recruitment of CD11b+ cells to 4T1 tumor cells at 8 hours. The effect on recruitment to B16F10 cells was less and not statistically significant (Figure 4A), although the number of CD11b+ cells recruited per tumor cell was decreased after treatment with the blocking antibody (data not shown). The more dramatic inflammatory response that 4T1 tumor cells exert in comparison with B16F10 cells could account for this difference. The populations of Gr-1+ and CD45+ cells recruited to 4T1 tumor cells were also reduced in the treated animals (supplemental Figure 7). Treatment with VCAM-1 blocking antibody did not significantly affect clot formation by the tumor cells (Figure 4B). Tumor cell survival in the lungs at 24 hours was reduced by 45% after treatment with VCAM-1 blocking antibody in both models (Figure 4C-D). To confirm that the VCAM-1 blocking antibody was functional in vivo, we noted that treatment with this antibody increased the levels of immature neutrophils in peripheral blood by promoting their release from the bone marrow, as previously reported (supplemental Figure 8A).38 Hence, inhibition of endothelial activation results in a moderate reduction in the early recruitment of myeloid cells and impaired tumor cell survival in the pulmonary vasculature.
Inhibition of endothelial activation decreases pulmonary metastasis. BALB/c, C57BL/6, or SCID mice were treated with VCAM-1 blocking antibody, VAP-1 inhibitor, or both. Mice were injected intravenously with 5 × 105 4T1-GFP, CMFDA-stained B16F10, or 1205Lu-GFP tumor cells, respectively. Lungs were harvested 8 hours later to assess for CD11b+ cell recruitment (A) and for clot formation (B), which were analyzed by immunohistochemistry against CD11b and the platelet-specific integrin αIIb, respectively. Lungs were harvested 24 hours after the introduction of tumor cells to analyze tumor cell survival, scored in lung sections (C; ≥ 10 sections analyzed per mouse) or by whole lung imaging, (D, as described in the supplemental Methods). Data represent mean + SD; n ≥ 3 mice (one-way analysis of variance and the Tukey test or Mann-Whitney test when only 2 groups were compared, for each cell line) and *P < .05; **P < .01; ***P < .001.
Inhibition of endothelial activation decreases pulmonary metastasis. BALB/c, C57BL/6, or SCID mice were treated with VCAM-1 blocking antibody, VAP-1 inhibitor, or both. Mice were injected intravenously with 5 × 105 4T1-GFP, CMFDA-stained B16F10, or 1205Lu-GFP tumor cells, respectively. Lungs were harvested 8 hours later to assess for CD11b+ cell recruitment (A) and for clot formation (B), which were analyzed by immunohistochemistry against CD11b and the platelet-specific integrin αIIb, respectively. Lungs were harvested 24 hours after the introduction of tumor cells to analyze tumor cell survival, scored in lung sections (C; ≥ 10 sections analyzed per mouse) or by whole lung imaging, (D, as described in the supplemental Methods). Data represent mean + SD; n ≥ 3 mice (one-way analysis of variance and the Tukey test or Mann-Whitney test when only 2 groups were compared, for each cell line) and *P < .05; **P < .01; ***P < .001.
PRX.A is a reversible small molecule inhibitor of the SSAO activity of VAP-1. PRX.A administration results in free plasma concentrations that would be expected to be sufficient to exceed 90% inhibition of the enzyme in vitro for at least 5 hours. Ex vivo assay of the fat SSAO activity in these mice confirmed that the enzyme was inhibited by a minimum of 60% (supplemental Figure 8B). Inhibition of VAP-1 resulted in a reduced recruitment of CD11b+ cells to 4T1 and to B16F10 tumor cells but not to 1205Lu tumor cells (Figure 4A). These results reveal an important role for VAP-1 in the recruitment of myeloid cells to tumor cells in the metastatic process. VAP-1 inhibition also reduced the recruitment of Gr-1+ and CD45+ cells to 4T1 tumor cells (supplemental Figure 7). VAP-1 inhibition had a slight inhibitory effect on the formation of clots by 4T1 cells and no effect on clot formation around B16F10 or 1205Lu cells (Figure 4B). VAP-1 inhibition decreased the survival of 4T1 and B16F10 tumor cells in the lungs at 24 hours but did not have an effect on the survival of 1205Lu tumor cells (Figure 4C-D), correlating with the reduction in the recruitment of monocytes/macrophages to 4T1 and B16F10 tumor cells after treatment. In conclusion, inhibition of either VCAM-1 or VAP-1 decreases the recruitment of monocytes/macrophages to the tumor cells and tumor cell survival.
Simultaneous inhibition of VCAM-1 and VAP-1 was not greater than VAP-1 inhibition alone on myeloid cell recruitment, tumor cell survival, or lung colony formation (Figure 4A-C). These results indicate that VCAM-1 and VAP-1 exert their effects on myeloid cell recruitment and subsequent tumor cell survival through closely related mechanisms.
Endothelial activation is essential for the early steps of metastasis but is dispensable afterward
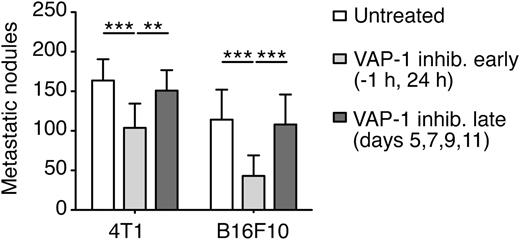
To investigate in which steps of the metastatic process endothelial activation was required, we inhibited VAP-1 at different times: from the initial steps of metastasis (1 hour before and 24 hours after introduction of the tumor cells) or after several days, when the tumor cells began to proliferate and establish colonies. In both models, 4T1 and B16F10, lung colony formation was decreased when the VAP-1 inhibitor was administered from the beginning of the experiment, but not when it was given 5 days after the injection of the tumor cells (Figure 5). This finding demonstrates that endothelial activation plays a role in the early steps of metastasis but is dispensable afterward.
VAP-1 activity is essential at the early steps of metastasis but dispensable afterward. BALB/c or C57BL/6 mice were treated with VAP-1 inhibitor either 1 hour before and 24 hours after the intravenous injection of 5 × 105 4T1-GFP (left) or B16F10 (right) tumor cells, respectively, (VAP-1 inhibition early), or on days 5, 7, 9, and 11 after the introduction of the tumor cells (VAP-1 inhibition late). Lungs were isolated on day 14 and the number of metastatic lung nodules was analyzed. Data represent mean + SD; n ≥ 10 mice (one-way analysis of variance and Tukey test) and **P < .01; ***P < .001.
VAP-1 activity is essential at the early steps of metastasis but dispensable afterward. BALB/c or C57BL/6 mice were treated with VAP-1 inhibitor either 1 hour before and 24 hours after the intravenous injection of 5 × 105 4T1-GFP (left) or B16F10 (right) tumor cells, respectively, (VAP-1 inhibition early), or on days 5, 7, 9, and 11 after the introduction of the tumor cells (VAP-1 inhibition late). Lungs were isolated on day 14 and the number of metastatic lung nodules was analyzed. Data represent mean + SD; n ≥ 10 mice (one-way analysis of variance and Tukey test) and **P < .01; ***P < .001.
Discussion
We have focused this study on 3 important aspects. First, we have identified endothelial activation molecules induced early during the process of pulmonary metastasis. Second, we have analyzed factors regulating their expression. Third, we have examined whether inhibition of endothelial activation could decrease metastasis. Our experiments demonstrate that VCAM-1 and VAP-1 are induced in the endothelium in the vicinity of tumor cells after their attachment to the vascular bed. VCAM-1 induction is enhanced by tumor cell clot formation but is independent of monocytes/macrophages recruitment. We further show that blocking endothelial activation, either with an anti-VCAM-1 blocking antibody or with a VAP-1 inhibitor, leads to reduced recruitment of myeloid cells and diminished tumor cell survival and metastasis. These experiments suggest a means for therapeutic intervention to combat early metastasis.
Recruitment of myeloid cells is well known to be required for early metastasis in many experimental systems.21,39 Our previous work demonstrated that coagulation is required for the recruitment of myeloid cells to arrested tumor cells.19 In our model, coagulation was triggered by expression of TF by tumor cells, and this clot then recruited myeloid cells. MCP-1/CCL2 production by tumor cells also has been found to recruit myeloid cells.22 Whether coagulation and MCP-1 are redundant or both are required is not known. In both of these models, ablation of the myeloid cells or inhibition of their recruitment led to failure of metastasis. Thus, the recruitment of myeloid cells can be a critical component of the metastatic process.
Homing of myeloid cells to inflamed tissues involves endothelial activation, leading to the question of whether myeloid recruitment by tumor cell induced clots also uses this pathway. We found evidence for aspects of endothelial activation, such as expression of VCAM-1 and VAP-1, but only inconsistently increased expression of E-selectin. VCAM-1 was induced by syngeneic cell lines: 4T1 in immunocompetent BALB/c mice, B16F10 in C57BL/6 mice, and Met-1 in FVB mice or by human cell lines, 1205Lu and MDA-MB-231, in immunocompromised (SCID) mice. There was little evidence of VCAM-1 expression before the tumor cells were introduced. Moreover, inhibition of VCAM-1 with a blocking antibody introduced before the tumor cells did not reduce the number of tumor cells attached to the pulmonary vasculature, in agreement with previous reports (Okahara et al24 and data not shown). However, VCAM-1 clearly was up-regulated in the endothelium adjacent to the tumor cells after attachment and persisted as microscopic colonies formed. These data suggest that VCAM-1 has functions in the process of metastasis other than initial adhesion of tumor cells to the endothelium.
The inconsistent involvement of E-selectin is reflected in the literature. Läubli et al40 found that E-selectin was up-regulated by MC38 murine colon adenocarcinoma cells, although E-selectin deficiency did not block metastasis. However, E-selectin inhibition attenuates liver metastasis in other models.40-42 We did not evaluate the potential roles of the endogenously expressed ICAM-1 and P-selectin.
We investigated factors regulating the expression of endothelial activation molecules in this system. Mac1 KO mice have diminished recruitment of monocytes/macrophages, yet the expression of VCAM-1 was not altered in this model. Hence, recruited macrophages do not appear to amplify endothelial activation. We asked whether tumor cell-induced coagulation was a regulator of endothelial activation. We used 2 different strategies to decrease clot formation directed by tumor cells, and they both decreased expression of VCAM-1 in the proximity of the tumor cells, implicating clot formation in the induction of VCAM-1. The tumor cell line 1205Lu, which does not express TF, did not exhibit extensive clot formation or induction of VCAM-1 expression.
TF expressed by leukocytes promotes systemic and local inflammation in mouse models of sickle cell disease. Inhibition of TF in sickle cell disease models reduced the plasma levels of soluble VCAM-1 and not those of soluble ICAM-1 or soluble E-selectin. This process was dependent on MCP-1 and KC.43 Administration of low-molecular-weight heparins reduces the plasma levels of soluble VCAM-1 to a similar extent.44 Continued VCAM-1 induction, on the other hand, appears coagulation independent and may be caused by cytokine production from the tumor cells themselves, although no single cytokine is apparent from the profiling. Thus, clot formation appears to be a significant contributor to the induction of endothelial VCAM-1 by tumor cells.
We then asked whether blockade of endothelial activation reduced the recruitment of myeloid cells to attached metastatic tumor cells and hence reduced the establishment of metastasis. To test the involvement of VCAM-1, we administered a blocking antibody. We observed a basal level of expression of VAP-1 in the quiescent murine lung, which is consistent with previous reports.45 The induction of VAP-1 is based upon both altered localization to the endothelial luminal surface and increased amounts at the transcriptional and translational levels. Thus, activation may occur without increased staining of VAP-1 by immunohistochemistry.3 Nevertheless, VAP-1 staining increased after tumor cell adhesion, peaking at 8 hours, coincident with the maxima for monocyte/macrophage recruitment.
To test the involvement of VAP-1 in metastasis, we resorted to the use of a small molecule inhibitor. Neither inhibition of VCAM-1 nor VAP-1 affected the extent of coagulation around the tumor cells, but both decreased the recruitment of myeloid cells and the survival of tumor cells. In Gil-Bernabé et al,19 we have shown that coagulation facilitates cell survival indirectly through the recruitment of myeloid cells. Here, we show that the recruitment and retention of myeloid cells is reduced by inhibition of the function of the endothelial activation molecules VCAM-1 and VAP-1, which at least in part is regulated by coagulation. In addition to adherence to endothelial activation markers, myeloid cells also have receptors that could allow them to bind to clots, both binding to fibrin and to urokinase.46,47 Inhibition of VCAM-1 and VAP-1 led to decreased myeloid cell retention, suggesting that endothelial activation plays a role in recruitment and leaving the significance of the potential homing to the clot components to be determined.
It should be noted that CD11b+ cell recruitment and tumor cell survival by the TF-deficient cell line 1205Lu was not affected by the VAP-1 inhibitor. Inhibition of both VCAM-1 and VAP-1 together had no greater effect than either alone, suggesting that they act on a single pathway. Neither affected clot formation. VAP-1 inhibition leads to reduced hydrogen peroxide production, a compound that has been reported to induce E- and P-selectins and VCAM-1.6,7 This finding suggests a possible mechanism explaining the nonadditive effect of VAP-1 and VCAM-1 inhibition. Although VAP-1 inhibition appeared more effective than VCAM-1 blocking antibody, it is hard to determine the efficacy of either, making this comparison uncertain. Inhibition of VAP-1 enzymatic activity is sufficient to reduce angiogenesis, tumor growth, and infiltration of CD11b+Gr-1+ myeloid cells into the tumors, being inhibition of its enzymatic activity sufficient for these effects.17,48 In our experiments with VAP-1 inhibitor, we find reduced metastatic cell survival and reduced myeloid cell recruitment. Because some metastatic cancer cells may use alternative mechanisms, stratification of patients would be essential for the clinical application of VAP-1 inhibition. Because VAP-1 inhibition, at least in mice, has only a slight decreased effect on immunity or defense against infection, the administration of VAP-1 inhibitor might be clinically feasible. The early phases of metastasis and not the later phases were affected, indicating that this target would be expected to be useful only in cases in which metastasis was being established, such as in the recurrence of cancer after treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Karla Watson and Magdalena Flieger (Gray Institute for Radiation Oncology and Biology, University of Oxford) for their assistance with in vivo experiments, Lei Zhao and Thomas Tapmeier (Gray Institute for Radiation Oncology and Biology, University of Oxford) for their help with flow cytometry, and Michael Ho and Leon Widdowson (Proximagen Group plc.) for performing VAP-1 activity assays on mouse fat tissue. They also thank Vittorio Katis for his help in the preparation of the manuscript.
This work was supported by Cancer Research U.K. and in part by Proximagen Group plc. R.J.M. is supported by Cancer Research U.K. and Medical Research Council.
Authorship
Contribution: Š.F. designed and performed research and analyzed and interpreted data; A.M.G.-B. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; S.A.H. provided expert assistance; P.D.A. analyzed data; P.R. and T.S. developed the VAP-1 inhibitor PRX.A; P.R. edited the manuscript; E.S. synthesized the VAP-1 inhibitor PRX.A; J.M. analyzed the pharmacokinetics and dosage of the VAP-1 inhibitor PRX.A; and R.J.M. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: P.R., T.S., E.S., and J.M. are employed by Proximagen Group plc., which supplied the VAP-1 inhibitor PRX.A and contributed to the consumables cost for the experiments. The remaining authors declare no competing financial interests.
Correspondence: Ruth J. Muschel, Gray Institute for Radiation Oncology and Biology, Old Road Campus Research Building, Roosevelt Dr, Oxford, OX3 7DQ, United Kingdom; e-mail: ruth.muschel@gmail.com.
References
Author notes
Š.F. and A.M.G.-B. contributed equally to this study.