Key Points
We conducted a phase 2 study of ATO followed by autologous HCT for relapsed APL.
This sequential treatment is effective and feasible.
Abstract
The optimal treatments for relapsed acute promyelocytic leukemia (APL) remain equivocal. We conducted a phase 2 study to evaluate the efficacy and feasibility of a sequential treatment consisting of induction and consolidation with arsenic trioxide (ATO), peripheral blood stem cell (PBSC) harvest after high-dose cytarabine chemotherapy, and autologous hematopoietic cell transplantation (HCT). Between 2005 and 2009, 35 patients (26 with hematologic and 9 with molecular relapse) were enrolled. Induction therapy resulted in complete remission in 81% of those with hematologic relapse, and most patients became negative for PML-RARα after the first ATO consolidation course, but 4 remained positive. Administration of the second ATO consolidation course further decreased the transcript levels in 3 patients. In total, 25 patients proceeded to PBSC harvest, all of whom successfully achieved the target CD34+ cell doses, and 23 underwent autologous HCT with PML-RARα–negative PBSC graft. Posttransplant relapse occurred in 3 patients, and there was no transplant-related mortality. With a median follow-up of 4.9 years, the 5-year event-free and overall survival rates were 65% and 77%, respectively. These findings demonstrate the outstanding efficacy and feasibility of the sequential treatment featuring ATO and autologous HCT for relapsed APL. This study was registered at http://www.umin.ac.jp/ctr/ as #C000000302.
Introduction
Outcomes for acute promyelocytic leukemia (APL) have improved significantly since the advent of all-trans retinoic acid (ATRA), and the recently introduced frontline therapy that combines ATRA and chemotherapy can provide long-term complete remission (CR) for a majority of patients with newly diagnosed APL.1-6 Nevertheless, relapse still occurs in ∼20% of cases, for which arsenic trioxide (ATO) has been shown to provide high CR rates exceeding 80%,7-9 thus making it a current recommendation for reinduction therapy.10,11 After returning to CR, autologous or allogeneic hematopoietic cell transplantation (HCT) for consolidating the CR status is generally considered if the patient is eligible for the procedure.10-12 However, because there have been few prospective studies for this very small patient population, the therapeutic approach after achievement of second or subsequent CR is mostly based on findings from retrospective studies.
In 2005, the Japan Adult Leukemia Study Group (JALSG) initiated a phase 2 study entitled APL205R for patients with relapsed APL. The main purpose of this study was to evaluate the efficacy and feasibility of a sequential treatment consisting of induction and consolidation with ATO, peripheral blood stem cell (PBSC) harvest after chemotherapy using high-dose cytarabine (AraC), and autologous HCT. This report presents and discusses the results of this study.
Methods
Patients
This study enrolled patients with relapsed APL between December 2005 and June 2009. At least a single documentation of cytogenetic and/or molecular evidence of t(15;17)/PML-RARα was required at the time of entry. Eligibility criteria consisted of age between 18 and 65 years; an Eastern Cooperative Oncology Group performance status between 0 and 3; and adequate functioning of the liver (serum bilirubin level <2.0 mg/L), kidneys (serum creatinine level <2.0 mg/dL), lungs (PaO2 ≥60 mm Hg or SpO2 ≥93%), and heart (no severe abnormalities detected on electrocardiograms). Patients who had previously undergone autologous or allogeneic HCT were not eligible for inclusion. Written informed consent was obtained from all patients prior to registration. The protocol was reviewed and approved by the institutional review board of each of the participating centers and was conducted in accordance with the Declaration of Helsinki. This study is registered at http://www.umin.ac.jp/ctr/ as #C000000302.
Treatments
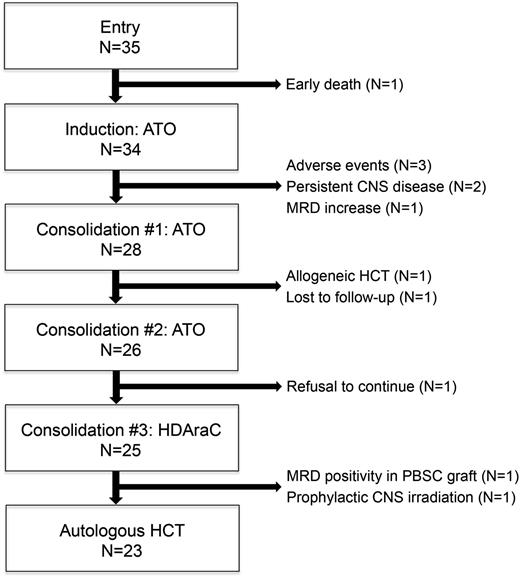
The treatments used during the study are summarized in Table 1. For remission induction, ATO was administered by a 2-hour infusion at a daily dose of 0.15 mg/kg until CR or a maximum of 60 days. In addition, patients received 12 mg/m2 of idarubicin (IDA) on days 1 and 2 if 1 or more of the following criteria were met when the treatment was started: (1) the white blood cell (WBC) count exceeded 20.0 × 109/L; (2) the combined total count of myeloblasts and promyelocytes in the peripheral blood exceeded 5.0 × 109/L; and (3) there was the presence of an extramedullary myeloid tumor. Patients who showed evidence of criteria 1 and/or 2 after the start of induction therapy were given 2 extra doses of 12 mg/m2 of IDA at that point. Those who achieved CR were scheduled to receive an additional 2 courses of ATO (0.15 mg/kg for 25 days) for consolidation. During ATO administration, a 12-lead electrocardiogram, complete blood cell counts, and chemistry parameters including the electrolytes were monitored at least twice a week, and the serum potassium and magnesium levels were maintained above the lower limits of normal. After the end of each ATO course, central nervous system (CNS) prophylaxis was attained by means of intrathecal injection of methotrexate, AraC, and corticosteroids (3 times in total). Patients with cytological evidence of CNS leukemia received intrathecal injections twice a week simultaneously with ATO, until complete clearance of leukemic cells in the cerebrospinal fluid (CSF) had been achieved. Following the third course of ATO, patients proceeded to PBSC harvest. For this purpose, high-dose AraC was administered at 2 g/m2 for 3 hours twice daily for 4 days, and granulocyte–colony-stimulating factor was initiated from day 6. Upon recovery, autologous PBSCs were harvested by means of apheresis. Patients who attained a target CD34+ cell dose of 2.0 × 106/kg or higher were allocated to undergo autologous HCT unless PML-RARα transcripts were detected in PBSCs. The conditioning regimen consisted of busulfan (1 mg/kg orally every 6 hours on days −6 to −4) and melphalan (70 mg/m2 intravenously on days −3 to −2),13 whereas unpurged autologous PBSCs were infused on day 0. The study flow is shown in Figure 1.
Treatment schedule
| Drug . | Dose . | Route . | Days . |
|---|---|---|---|
| Induction | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-* |
| IDA | 12 mg/m2 | IV (30 min) | † |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #1 | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-25 |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #2 | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-25 |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #3 | |||
| AraC | 2 g/m2, every 12 h | IV (3 h) | 1-4 |
| PBSCH | § | ||
| Autologous HCT | |||
| Busulfan | 1 mg/kg, every 6 h | po | −6, −5, −4 |
| Melphalan | 70 mg/m2 | IV (bolus) | −3, −2 |
| PBSCT | 0 |
| Drug . | Dose . | Route . | Days . |
|---|---|---|---|
| Induction | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-* |
| IDA | 12 mg/m2 | IV (30 min) | † |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #1 | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-25 |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #2 | |||
| ATO | 0.15 mg/kg | IV (2 h) | 1-25 |
| MTX, AraC, PSL | 15 mg, 40 mg, 10 mg | IT | ‡ |
| Consolidation #3 | |||
| AraC | 2 g/m2, every 12 h | IV (3 h) | 1-4 |
| PBSCH | § | ||
| Autologous HCT | |||
| Busulfan | 1 mg/kg, every 6 h | po | −6, −5, −4 |
| Melphalan | 70 mg/m2 | IV (bolus) | −3, −2 |
| PBSCT | 0 |
IT, intrathecally; IV, intravenously; MTX, methotrexate; PBSCH, peripheral blood stem cell harvest; PBSCT, peripheral blood stem cell transplantation; po, by mouth; PSL, prednisolone.
For induction, ATO was administered until complete remission or for 60 d, whichever was shorter.
IDA was added for 2 d if the WBC count exceeded 20.0 × 109/L before or during the induction therapy, if the combined total count of myeloblasts and promyelocytes in the peripheral blood exceeded 5.0 × 109/L before or during the induction therapy, or if an extramedullary myeloid tumor was detected before the induction therapy.
Intrathecal injection was given when the platelet count recovered after the end of the courses. PSL could be replaced with 4 mg of dexamethasone.
PBSCH was performed when the WBC count had recovered.
Patient flow diagram. HDAraC, high-dose cytarabine; MRD, minimal residual.
Assessments and definitions
Hematologic CR was defined as the presence of all of the following: <5% of blasts in the bone marrow, no leukemic blasts in the peripheral blood or extramedullary sites, and recovery of peripheral blood counts. Hematologic relapse was defined as the presence of at least 1 of the following: recurrence of >10% leukemic cells in the bone marrow, recurrence of any leukemic cells in the peripheral blood, or development of extramedullary disease.3 Molecular relapse was defined as the reappearance of polymerase chain reaction (PCR) positivity for PML-RARα in a single bone marrow or peripheral blood sample for this study. Prospective molecular monitoring was performed with the real-time quantitative reverse-transcription PCR (qRT-PCR) assay in a single independent laboratory. The PML-RARα levels in bone marrow samples were assessed at enrollment and after each course of therapy. Harvested PBSCs were also subjected to the qRT-PCR assay. The number of transcript copies was normalized by means of glyceraldehyde-3-phosphate dehydrogenase, and then converted into molecules per µg RNA. The threshold for quantification was 50 copies per µg RNA, which corresponds to a sensitivity of 10−4, whereas levels below the threshold were differentiated into “not detected” and “detected but not quantifiable,” and PCR negativity was categorized as “not detected.”
For posttransplant engraftment, neutrophil engraftment was defined as achievement of a neutrophil count of at least 0.5 × 109/L for 2 consecutive days, and platelet engraftment as achievement of a platelet count of at least 30 × 109/L independent of transfusions for 2 consecutive days.
Statistical analysis
The primary end point was event-free survival (EFS) at 1 year after registration, which was defined as the time from registration to failure to achieve CR, relapse, death, or last visit, whichever came first. The expected and threshold EFS rates at 1 year were estimated to be 50% and 20%, respectively. The threshold EFS rate of 20% was determined based on historical control data of Japanese patients with relapsed APL who were treated with ATRA-based therapy.14 With a statistical power of 80% and a 1-sided, type I error of 5%, the minimum number of 17 eligible patients required for this study was calculated by means of binomial analysis. Allowing for a premature dropout rate of 15%, we aimed for inclusion of at least 20 patients. Primary end point analysis was performed with the Kaplan-Meier method for the calculation of probability of EFS. The treatment was considered to be effective if the lower limit of the 90% confidence interval (CI) exceeded the threshold EFS (ie, 20%). Overall survival (OS) was defined as the time from registration to death or last visit, and failure-free survival as the time from registration to failure to achieve CR, withdrawal from study, relapse, death, or last visit. Survival estimates and CIs were calculated with the Kaplan-Meier method and Greenwood’s formula. The log-rank test was used for group comparison.
Results
Patient characteristics
A total of 35 patients with relapsed APL were enrolled in this study. Patient enrollment was allowed to exceed the originally planned minimum requirement after having ensured it ethical to expand the number of patients. Table 2 summarizes baseline characteristics of the patients. There were 23 males and 12 females, with a median age at enrollment of 46 years (range, 20-64 years). The median interval between primary diagnosis and enrollment was 2.5 years (range, 0.8-11.0 years).
Patient characteristics at enrollment
| Characteristics . | Values . |
|---|---|
| Age in years, median (range) | 46 (20-64) |
| Gender, male/female | 23/12 |
| WBC count, x 109/L | |
| Median (range) | 2.6 (0.5-18.1) |
| ≤10/>10 | 34/1 |
| Platelet count, x 109/L | |
| Median (range) | 79 (8-260) |
| ≤40/>40 | 9/26 |
| Performance status, 0/1/2/3 | 27/6/0/2 |
| Number of prior relapses, 1/2 | 32/3 |
| Type of relapse, hematologic/molecular | 26/9 |
| Interval between primary diagnosis and enrollment in years, median (range) | 2.5 (0.8-11.0) |
| Characteristics . | Values . |
|---|---|
| Age in years, median (range) | 46 (20-64) |
| Gender, male/female | 23/12 |
| WBC count, x 109/L | |
| Median (range) | 2.6 (0.5-18.1) |
| ≤10/>10 | 34/1 |
| Platelet count, x 109/L | |
| Median (range) | 79 (8-260) |
| ≤40/>40 | 9/26 |
| Performance status, 0/1/2/3 | 27/6/0/2 |
| Number of prior relapses, 1/2 | 32/3 |
| Type of relapse, hematologic/molecular | 26/9 |
| Interval between primary diagnosis and enrollment in years, median (range) | 2.5 (0.8-11.0) |
All of the patients had been initially treated with ATRA-based therapy, and most of them in accordance with the protocols of JALSG or modifications thereof.3,15 Thirty-two patients were in first relapse, and 3 in second relapse, with hematologic relapse accounting for 26, and molecular for 9. None of the patients had received ATO before.
Induction with ATO
ATO was administered to all patients except for 1 who developed intracranial hemorrhage immediately after enrollment and succumbed to early death (unique patient number [UPN] 26). Of the remaining 34 patients who underwent induction therapy, IDA was added for 2 patients on days 1 and 2, and during the induction course for 8 patients as per protocol. None of the patients developed differentiation syndrome. Three patients discontinued the study due to adverse events (grade 3 skin rash [UPN 10], grade 3 QT prolongation [UPN 19], and grade 4 QT prolongation accompanied by frequent ventricular premature contraction [UPN 23]). CSF examination performed at the end of the induction therapy revealed cytological evidence of CNS involvement in 4 patients, 2 of whom discontinued due to persistent CNS disease despite repeated intrathecal injections (UPN 13 and UPN 33). Of the 26 patients with hematologic relapse, 5 were taken off the study as mentioned previously, whereas the other 21 (81%) achieved CR. Of the 9 patients presenting with molecular relapse, 7 proceeded to consolidation therapy, and 2 were withdrawn from the study because of persistent CNS disease (UPN 13) or at the physician’s discretion because the PML-RARα levels increased significantly after induction therapy (UPN 29).
Consolidation with ATO
During the 2 consolidation courses with ATO, 3 patients were taken off the study: 1 discontinued the protocol after the first consolidation course to receive umbilical cord blood transplantation (UPN 1), 1 was lost to follow-up after completing the first consolidation course (UPN 14), and the other refused to continue for unknown reasons after the second consolidation course (UPN 30). None of the patients discontinued the study because of relapse or adverse events during this phase of the treatment.
High-dose AraC and PBSC harvest
For PBSC harvest, 25 patients were given high-dose AraC as the third consolidation therapy, and all of them attained the target CD34+ cell doses of 2.0 × 106/kg. The median value of the CD34+ cell doses was 6.5 × 106/kg (range, 2.0-42.2 × 106/kg). One patient (UPN 18) whose PBSC sample was positive for PML-RARα was taken off the study because of ineligibility for autologous HCT as per protocol. One other patient (UPN 3), who had documented CNS leukemia at the end of induction therapy, but whose leukemic cells in the CSF were completely cleared with intrathecal injections, was withdrawn from the protocol at the physician’s discretion to undergo prophylactic CNS irradiation. This patient received autologous HCT, but not as part of this study, and subsequently suffered posttransplant relapse in the CNS with fatal outcome. All of the other patients proceeded to autologous HCT. No dropouts due to relapse or adverse events were reported during this phase of the treatment.
Autologous HCT
The remaining 23 patients underwent autologous HCT as per protocol. The median time until engraftment was 12 days (range, 11-39 days) for neutrophils and 15 days (range, 12-136 days) for platelets. Posttransplant relapse occurred in 3 patients after a median duration of 5 months (range, 3-6 months). There was no transplant-related mortality.
Kinetics of the PML-RARα transcript levels
The results of the serial qRT-PCR tests during the treatment are summarized in Table 3. Most patients achieved PCR negativity after the first consolidation, but 4 were still positive for PML-RARα at this time. The PCR results turned negative after the second and third consolidation in 1 patient each (UPN 25 and 17, respectively). Of the 2 patients who remained positive for PML-RARα after the third consolidation, 1 (UPN 18) showed positive and the other (UPN 5) negative PCR test results for PBSCs. The latter underwent autologous HCT with a PML-RARα–negative graft but relapsed 5 months after transplantation.
Kinetics of PML-RARα transcript levels
| UPN . | At entry . | After induction . | After consolidation #1 . | After consolidation #2 . | After consolidation #3 . |
|---|---|---|---|---|---|
| 1 | 3000 | N | N | Off study | Off study |
| 2 | 460 | N | N | N | N |
| 3 | 60 000 | <50 | N | N | N |
| 4 | 4200 | <50 | N | N | NA |
| 5 | 69 000 | 28 000 | 760 | 140 | <50 |
| 6 | 32 000 | 6000 | N | N | N |
| 7 | 15 000 | 290 | N | N | N |
| 8 | 360 000 | <50 | N | N | N |
| 9 | NA | 1000 | N | NA | N |
| 10 | NA | Off study | Off study | Off study | Off study |
| 11 | 950 | N | NA | N | N |
| 12 | 64 000 | 50 | N | NA | N |
| 13 | 10 000 | 7100 | Off study | Off study | Off study |
| 14 | 120 000 | 400 | NA | Off study | Off study |
| 15 | 510 000 | 150 | N | N | NA |
| 16 | 190 000 | <50 | N | N | NA |
| 17 | 95 000 | 1800 | 110 | 110 | N |
| 18 | 67 000 | 1500 | 480 | 390 | 280 |
| 19 | 130 000 | Off study | Off study | Off study | Off study |
| 20 | 450 000 | 280 000 | N | N | N |
| 21 | 140 000 | 170 | N | N | N |
| 22 | 26 000 | 61 | N | N | N |
| 23 | 24 000 | Off study | Off study | Off study | Off study |
| 24 | 730 000 | <50 | N | N | N |
| 25 | 1900 | 2500 | <50 | N | N |
| 26 | 440 000 | Off study | Off study | Off study | Off study |
| 27 | NA | 7800 | N | N | N |
| 28 | NA | 2600 | N | N | N |
| 29 | 510 | 6300 | Off study | Off study | Off study |
| 30 | 45 000 | 65 | N | N | Off study |
| 31 | NA | 300 000 | NA | N | N |
| 32 | NA | 50 | N | N | N |
| 33 | 180 000 | NA | Off study | Off study | Off study |
| 34 | 20 000 | N | N | N | N |
| 35 | 150 000 | 10 000 | N | N | N |
| UPN . | At entry . | After induction . | After consolidation #1 . | After consolidation #2 . | After consolidation #3 . |
|---|---|---|---|---|---|
| 1 | 3000 | N | N | Off study | Off study |
| 2 | 460 | N | N | N | N |
| 3 | 60 000 | <50 | N | N | N |
| 4 | 4200 | <50 | N | N | NA |
| 5 | 69 000 | 28 000 | 760 | 140 | <50 |
| 6 | 32 000 | 6000 | N | N | N |
| 7 | 15 000 | 290 | N | N | N |
| 8 | 360 000 | <50 | N | N | N |
| 9 | NA | 1000 | N | NA | N |
| 10 | NA | Off study | Off study | Off study | Off study |
| 11 | 950 | N | NA | N | N |
| 12 | 64 000 | 50 | N | NA | N |
| 13 | 10 000 | 7100 | Off study | Off study | Off study |
| 14 | 120 000 | 400 | NA | Off study | Off study |
| 15 | 510 000 | 150 | N | N | NA |
| 16 | 190 000 | <50 | N | N | NA |
| 17 | 95 000 | 1800 | 110 | 110 | N |
| 18 | 67 000 | 1500 | 480 | 390 | 280 |
| 19 | 130 000 | Off study | Off study | Off study | Off study |
| 20 | 450 000 | 280 000 | N | N | N |
| 21 | 140 000 | 170 | N | N | N |
| 22 | 26 000 | 61 | N | N | N |
| 23 | 24 000 | Off study | Off study | Off study | Off study |
| 24 | 730 000 | <50 | N | N | N |
| 25 | 1900 | 2500 | <50 | N | N |
| 26 | 440 000 | Off study | Off study | Off study | Off study |
| 27 | NA | 7800 | N | N | N |
| 28 | NA | 2600 | N | N | N |
| 29 | 510 | 6300 | Off study | Off study | Off study |
| 30 | 45 000 | 65 | N | N | Off study |
| 31 | NA | 300 000 | NA | N | N |
| 32 | NA | 50 | N | N | N |
| 33 | 180 000 | NA | Off study | Off study | Off study |
| 34 | 20 000 | N | N | N | N |
| 35 | 150 000 | 10 000 | N | N | N |
“Off study” indicates that the patient discontinued the study for reasons detailed in the text.
The threshold for quantification was 50 copies per µg RNA, which corresponds to a sensitivity of 10−4. The levels below the threshold were differentiated into “not detected (N)” and “detected but not quantifiable (<50).”
N, not detected; NA, not assessed.
Overall outcome
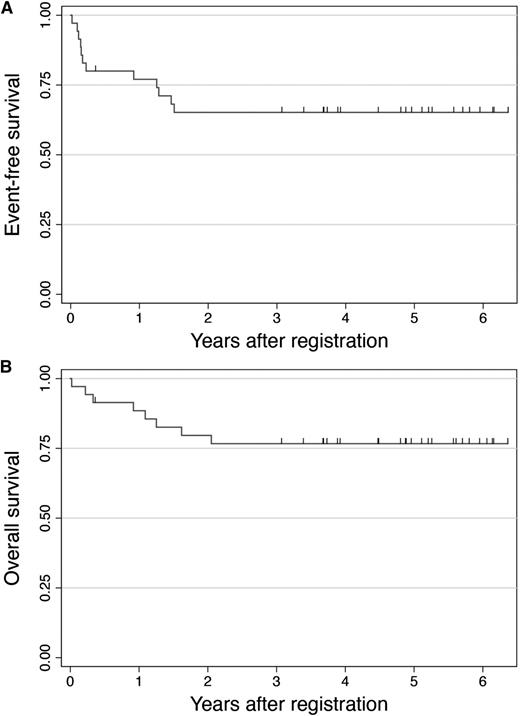
The probability of EFS was 77% at 1 year, with the 90% CIs ranging from 63% to 86%, thus demonstrating that this study has met its primary end point. Figure 2 shows Kaplan-Meier estimates for EFS and OS. With a median follow-up for surviving patients of 4.9 years (range, 0.3-6.3 years), the 5-year EFS and OS rates were 65% and 77%, respectively. The probability of failure-free survival was estimated to be 59% at 5 years.
Kaplan-Meier curves for EFS (A) and OS (B). The probabilities of EFS and OS for the entire cohort (N = 35) were 65% and 77% at 5 years, respectively.
Kaplan-Meier curves for EFS (A) and OS (B). The probabilities of EFS and OS for the entire cohort (N = 35) were 65% and 77% at 5 years, respectively.
Discussion
Current comprehensive practice guidelines have provided recommendations on the management of APL,10,11 but what the optimal treatments for relapsed APL are remains equivocal. This is primarily because of the lack of prospective studies due to the rarity of relapses in APL, so that we initiated a phase 2 study for patients with relapsed APL in 2005 to evaluate the efficacy and feasibility of a sequential treatment featuring ATO and autologous HCT and enrolled 35 patients from 25 institutions nationwide. The treatment immediately induced molecular remission in a majority of patients, and only 3 patients were taken off the protocol because of adverse events throughout the entire study period, so that 23 patients could receive autologous HCT with a PML-RARα–negative PBSC graft. The 5-year probabilities of EFS and OS for the entire cohort were 65% and 75%, respectively. Of note, the EFS curve reached a stable plateau after 2 years from registration. These results have led us to conclude that this sequential treatment is effective and feasible.
ATO is currently the most active agent available for APL. Accumulated evidence has shown that >80% of patients with relapsed APL can achieve CR with ATO monotherapy.7-9 In addition to high CR rates, the capability of this agent to induce molecular remission is another significant advantage because molecular remission is a prerequisite for long-term disease control in APL and is thus considered an important therapeutic milestone.10,16 By contrast, ATRA alone is less likely to induce molecular remission, which results in this agent being used generally in combination with intensive chemotherapy rather than as monotherapy.17 Although high CR rates can be expected for such combined use, this approach is limited by unsustained CR, especially for patients with hematologic relapse, and, more importantly, by quite high toxicity.18,19 A retrospective study by Thomas et al18 reported better survival for relapsed APL patients treated with ATO-based therapy than for historical control patients treated with ATRA-based therapy. The favorable safety profile of ATO is also an important advantage, as was seen in our study, where only 3 patients (8%) had to discontinue the protocol because of adverse events during induction therapy. This ratio seems to be only slightly higher than that observed in a US Intergroup study (5%).8 It is further worth noting that none of our patients developed differentiation syndrome. This contrasts with a high incidence of this complication (25%) in the American study.8 It can be assumed that the additional use of IDA for cases with high WBC counts may have contributed to reducing the risk of differentiation syndrome in our cohort.
Although the beneficial effect of ATO for induction has been well documented in relapsed APL, it is far less clear what the best consolidation strategy is after achieving CR. Previous studies showed that patients who achieved second or subsequent CR with ATO but did not receive transplantation thereafter had poor outcome; the proportion of those remaining alive and relapse-free ranged from 22% to 37%.7,17,20 Although some patients may remain in CR without transplantation, overall prognosis is far from satisfactory, and the outcome seems much better for those who receive autologous or allogeneic HCT.17,20 Owing to its posttransplant graft-versus-leukemia effect, allogeneic HCT is generally considered the most effective treatment of preventing relapse in acute myeloid leukemia.21 In APL, however, the relapse rate after autologous HCT may be quite low provided the patient is in molecular remission at the time of transplantation.22-25 Given the lower risk of transplant-related mortality with autologous HCT, the balance of benefits and risks may well favor autologous HCT over allogeneic HCT. For autologous HCT to be successful, it is imperative to reduce the tumor burden substantially at the molecular level before transplantation. For this reason, what constitutes an adequate number of cycles of ATO therapy is a subject of clinical interest. Similar to the observation by the US Intergroup,8 our study found that 2 courses of ATO therapy induced most patients into molecular remission, although 4 patients remained positive for PML-RARα after the second course (ie, consolidation #1). Administration of the third ATO course reduced the transcript levels in 3 of the patients, whereas the level stayed unchanged in the remaining patient. It was possible to administer the third course of ATO because none of the 26 patients who had received this course had to withdraw from the study due to relapse or adverse events. These findings lead us to consider that administration of a total of 3 courses of ATO before PBSC collection is feasible.
For the PBSC-mobilizing regimen, we chose high-dose AraC, hoping it would produce highly efficient mobilization as well as exert a systemic antileukemic effect. The fact that all the 25 patients undergoing this procedure successfully achieved the target CD34+ cell doses has convinced us of the usefulness of this regimen. In addition, high-dose AraC is known to provide good coverage of the CNS, the most common site of extramedullary involvement in APL.26,27 Above and beyond our expectations, routine CSF examination at the end of the induction therapy identified 4 patients with cytological evidence of CNS involvement, although they did not show any CNS-related symptoms. This suggests that high-dose AraC may also play a part in protecting against the potential risk of subsequent CNS relapse for these patients.
Except for 1 patient whose PBSC sample was positive for PML-RARα and another who was withdrawn from the study to receive off-protocol prophylactic CNS irradiation, all the remaining patients who had undergone PBSC harvest proceeded to autologous HCT without any subsequent transplant-related mortality. This contrasts with a previous prospective study conducted before the advent of ATO, in which a combination of ATRA and intensive chemotherapy was used.28 In that study, severe toxicity of induction therapy precluded the subsequent conduct of PBSC harvest or autologous HCT for some patients, and nearly 10% of the autografted patients suffered transplant-related mortality. These results highlight the need for active and less toxic therapies that give patients a better chance to proceed to and receive autologous HCT safely. For this reason, ATO can be considered to be an ideal treatment because of its strong antileukemic effect and favorable safety profile.
Although relatively few patients were analyzed in our study, to our knowledge this is the first prospective study to evaluate the use of ATO in conjunction with autologous HCT for relapsed APL. The results presented here provide evidence of the outstanding efficacy and feasibility of the sequential treatment consisting of induction and consolidation with ATO, PBSC harvest after high-dose AraC chemotherapy, and autologous HCT. For patients who are not eligible for this strategy, such as those for whom autologous HCT is not suitable or whose PML-RARα levels do not decrease sufficiently during treatment, other treatment approaches need to be investigated that incorporate, for example, allogeneic HCT,24,29 gemtuzumab ozogamicin,30,31 tamibarotene,32 or novel agents. It is desirable that such studies can be conducted prospectively. Finally, we should remember that the incorporation of ATO into initial therapy is expected to further improve the outcome for newly diagnosed APL,33,34 which will hopefully lead to reduction in the number of patients who require salvage therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all physicians and staff at the JALSG participating centers.
This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (Clinical Cancer Research 23-004) and the National Cancer Center Research and Development Fund (23-A-23).
Authorship
Contribution: M.Y. collected and analyzed data, interpreted results, and drafted the manuscript; M.T., H.F., and A.T. designed the study, collected data, interpreted results, and reviewed the manuscript; K.F., S.F., K.S., M.T., A.O., K.T., and A.M. collected data, interpreted results, and reviewed the manuscript; S.O. contributed to data management, designed the study, collected data, interpreted results, and reviewed the manuscript; Y.M. contributed to data management, interpreted results, and reviewed the manuscript; Y.A. designed the study, analyzed data, interpreted results, and drafted the manuscript; Y.K. designed the study, provided administrative support, interpreted results, and reviewed the manuscript; T.N. provided administrative support, interpreted results, and reviewed the manuscript; and N.E. served as the principal investigator, designed the study, collected and analyzed data, interpreted results, and drafted the manuscript.
Conflict-of interest-disclosure: The authors declare no competing financial interests.
A complete list of the members of the JALSG appears in “Appendix: study group members.”
Correspondence: Masamitsu Yanada, Department of Hematology, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukake-cho, Toyoake, 470-1192, Japan; e-mail: myanada@fujita-hu.ac.jp.
Appendix: study group members
The members of the JALSG are Nihon University School of Medicine, Kasukabe Municipal Hospital, Tokyo Metropolitan Komagome Hospital, Tokyo Metropolitan Ohtsuka Hospital, Nagoya University Graduate School of Medicine, Nagoya Ekisaikai Hospital, JA Aichi Showa Hospital, Okazaki City Hospital, Daido Hospital, Yokkaichi Municipal Hospital, Ichinomiya Municipal Hospital, Komaki City Hospital, Toyohashi Municipal Hospital, Ogaki Municipal Hospital, Tosei General Hospital, National Center for Geriatrics and Gerontology, Aichi Cancer Center, Toyota Kosei Hospital, Japanese Red Cross Nagoya First Hospital, Fujita Health University School of Medicine, Mie University Graduate School of Medicine, Suzuka Kaisei Hospital, Takeuchi Hospital, Yamada Red Cross Hospital, JA Suzuka General Hospital, Matsusaka Chuo General Hospital, Kinki University School of Medicine, Osaka Minami Medical Center, Sakai Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, Hiroshima Red Cross Hospital & Atomic-Bomb Survivors Hospital, Shikoku Cancer Center, Nagasaki University Graduate School of Biomedical Sciences, Sasebo City General Hospital, Nagasaki Medical Center, Kumamoto University School of Medicine, Kumamoto City Hospital, Kumamoto Shinto General Hospital, Jichi Medical School, Okayama University Hospital, Minami-Okayama Medical Center, Okayama City Hospital, Chugoku Central Hospital, Okayama Medical Center, Okayama Rosai Hospital, Kagawa Rosai Hospital, Gunma University Graduate School of Medicine, Nishi-Gunma National Hospital, Fujioka General Hospital, Fukaya Red Cross Hospital, University of Fukui, Kurashiki Central Hospital, Kanazawa Medical Center, Fukui Red Cross Hospital, Fukui Prefectural Hospital, National Cancer Center Hospital, Saitama Medical School, Hyogo College of Medicine, Osaka National Hospital, Takarazuka Municipal Hospital, Uegahara Hospital, Amagasaki Central Hospital, Kawasaki Medical School, Kochi Health Sciences Center, Chiba University Hospital, Chiba Aoba Municipal Hospital, Funabashi Central Hospital, Saiseikai Narashino Hospital, Oami Hospital, Nara Medical University, Jikei University School of Medicine, Dokkyo University School of Medicine, Nagoya Medical Center, Ohta Nishinouchi Hospital, Kochi Medical School, Shiga University of Medical Science, National Cancer Center East, Anjo Kosei Hospital, St. Marianna University School of Medicine, Yokohama Seibu Hospital, Shinshu University School of Medicine, Nagano Red Cross Hospital, Matsumoto Medical Center Matsumoto Hospital, Showa Inan General Hospital, Tokyo Women's Medical University, Tama-Hokubu Medical Center, Hamamatsu University School of Medicine, Hamamatsu Medical Center, Kagoshima University Hospital, Tochigi Cancer Center, Kanazawa University Graduate School of Medical Science, Keijyu Medical Center, NTT West Kanazawa Hospital, Toyama City Hospital, Ishikawa Central Hospital, JA Takaoka Hospital, Tokyo Medical University, Tokyo Medical University Hachioji Medical Center, Kyorin University School of Medicine, Hokkaido University Graduate School of Medicine, Sapporo Kousei Hospital, Sapporo Aiiku Hospital, Asahikawa City Hospital, Hakodate City Hospital, Hokkaido Cancer Center Hospital, Saiseikai Maebashi Hospital, Nagoya City University Graduate School of Medical Sciences, Enshu General Hospital, Shizuoka Saiseikai General Hospital, Tokai University School of Medicine, Ebina General Hospital, Yamaguchi University School of Medicine, Yamaguchi Prefecture Central Hospital, The University of Tokyo, Osaka City University, Saiseikai Nakatsu Hospital, Osaka University Graduate School of Medicine, University of Tokyo, Niigata University Medical and Dental Hospital, Oita University Faculty of Medicine, Oita Prefectural Hospital, Almeida Memorial Hospital, Kouseiren Tsurumi Hospital, National Kyushu Cancer Center, Kyushu Medical Center, Fukuoka Postal Services Agency Hospital, Aso Iizuka Hospital, Teikyo University School of Medicine, Teikyo University Mizonokuchi Hospital, Sapporo Hokuyu Hospital, Aichi Medical University, Kitasato University Hospital, Yamagata University Faculty of Medicine, Keio University, Aomori Prefectural Central Hospital, Hyogo Cancer Center, Kyoto Prefectural University of Medicine, Kyoto Hospital, Kobe Central Hospital, Matsushita Memorial Hospital, Osaka City General Hospital, National Defense Medical College, Akita University School of Medicine, NTT Kanto Medical Center, Yokohama City University Hospital, Yokohama City University Medical Center, Kanagawa Cancer Center, Yokosuka City Hospital, Fujisawa City Hospital, Shizuoka Red Cross Hospital, Yamato Municipal Hospital, Saiseikai Yokohama Nanbu Hospital, Tohoku University School of Medicine, Osaki Citizen Hospital, Hiroshima University, Hiroshima-Nishi Medical Center, Kagawa University, Kagawa Prefectural Central Hospital, Sakaide City Hospital, Juntendo University School of Medicine, Kanazawa Medical University, Kobe University Graduate School of Medicine, Imamura Bun-In Hospital, Ehime University School of Medicine, Bokutoh Hospital, Ohtsu Red Cross Hospital, Matsue Red Cross Hospital, Tokyo Medical and Dental University, Yokohama City Minato Red Cross Hospital, Jichi Medical School Omiya Medical Center, Shizuoka Cancer Center Hospital, Ehime Prefectural Central Hospital, International Medical Center of Japan, Kure Medical Center, Nagoya Daini Red Cross Hospital, University of Yamanashi, Heart Life Hospital, Musashino Red Cross Hospital, Saitama Medical School, PL General Hospital, Toyama Prefectural Central Hospital, Shimane Prefectural Central Hospital, Tottori Prefectural Central Hospital, National Disaster Medical Center, Shimane University Hospital, Otemae Hospital, Nakagami Hospital, Tsukuba University Hospital, Mito Medical Center, National Hospital Organization, Tsuchiura Kyodo General Hospital, Hitachi General Hospital, JA Toride Medical Center, Fuchu Hospital, Ibaraki Prefectural Central Hospital, Saga University, Yamanashi Prefectural Central Hospital, Hiroshima City Asa Hospital, Sendai Medical Center, Kurume University of Medicine, Kitakyushu Municipal Medical Center, Kyushu Kosei-Nenkin Hospital, University of Miyazaki Hospital, Hamanomachi Hospital, Kyushu University Graduate School of Medical Sciences, Harasanshin Hospital, Toranomon Hospital, Matsuyama Red Cross Hospital, Fukushima Medical University, Fukuoka University Hospital, Yokohama Municipal Citizen's Hospital, Nagoya City West Medical Center, Takanohara Central Hospital, The University of the Ryukyus, Osaka Red Cross Hospital, and Sapporo Medical University School of Medicine.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal