Key Points
Using B cells to target antigens into the follicular regions represents a novel approach to accelerate CD8+ T-cell recall responses.
Abstract
Rapid boosting of memory CD8+ T cells (TM) is essential in cancer immunotherapy and the control of certain infectious diseases. However, effector T cells (TE) are a barrier to booster vaccination because they can rapidly kill antigen-bearing antigen-presenting cells (APCs) before TM are engaged. We demonstrate that viral-vectored vaccines delivered by B cells elicit robust TM expansion in the presence of TE, enabling booster immunizations to bypass TE-mediated negative feedback regulation. Our data indicate that viral vector–loaded B cells home to the follicular regions in secondary lymphoid organs, which are anatomically separated from TE and in close proximity to TM. The B cells, however, do not serve as APCs in this area. Rather, classic CD11c+ dendritic cells serve to stimulate the secondary CD8+ T-cell response. Our data reveal that B cells represent a novel and readily accessible delivery system that can effectively engage secondary CD8+ T-cell activation for prime-boost strategies.
Introduction
CD8+ cytotoxic T lymphocytes (CTL) are essential for immune protection against intracellular pathogens and tumors. Various forms of vaccines have been designed specifically to elicit CTL responses. In particular, viral vectors are being widely studied for use in gene-based vaccine strategies because of their ability to deliver antigens into the antigen-processing pathways needed to stimulate major histocompatibility complex (MHC) class I–restricted CTL responses.1,2 Furthermore, the frequency of transgene-specific T cells can be further increased by combining recombinant viral vectors in heterologous prime-boost regimens.3,4 A central role for dendritic cells (DCs) in priming CD8+ T cells has been well-established following viral infection or vaccination.5,6 This includes the transportation of viral antigens by migratory DCs to draining lymph nodes or spleen and subsequent antigen presentation by lymphoid residential DCs.7,8 Upon activation, effector and effector memory T cells enter the circulation and peripheral tissues, whereas central memory T cells home to the secondary lymphoid organs.9-11 This differential distribution suggests that, similar to the priming process, booster amplification of central memory T cells also requires cooperation between migratory and residential DCs. However, acceleration of CD8+ T-cell boosting may be limited by circulating CTL that can readily kill migratory DCs in peripheral tissues and prevent their interaction with central memory T cells.12 Indeed, we and others have provided evidence that readministered DC vaccines fail to reach lymphoid organs in the presence of vigorous CTL response.13-15
As another major subset of APCs, B cells share many biological features with DCs, such as a high level of MHC expression and the capacity to produce cytokines that enable them to regulate antigen-specific T-cell responses. Thus, there is an increasing interest in using B cells as an alternative to DCs as cell-based vaccines for immunotherapy.16,17 In particular, large numbers of autologous B cells can be readily prepared from the blood of patients and further expanded ex vivo, providing an additional advantage over DCs, which are not readily expanded when differentiated from monocytes, to meet therapeutic schedules where multiple vaccinations are required.18,19 Furthermore, intravenously administered B cells readily migrate to secondary lymphoid organs, offering a potentially more effective platform to deliver antigens to the primary site of T-cell activation. However, whether B cell–based vaccines can escape CTL-mediated elimination during booster immunization has not been studied.
In the present study, we compared the capacity of ex vivo–generated DCs and B cells to elicit secondary CD8+ T-cell expansion following in vitro exposure to viral-vectored vaccines. Our data indicate that only vector-exposed B cells, but not DCs, could trigger a secondary CD8+ T-cell response during primary T-cell activation, identifying B cells as a unique vaccination platform to shorten the interval between the prime and boost. Furthermore, B cells remained more potent than DCs even at a later time point when circulating effector T cells declined, indicating that B cells are generally superior to DCs for eliciting a recall response. We demonstrated that B cells did not present antigens themselves. Rather, the B cells delivered vectored vaccines to the follicular regions in secondary lymphoid organs where memory T cells are located. This unique property does not only allow B-cell carriers to bypass initial CTL killing but also led to antigen presentation by host CD11c+ APCs within follicular areas where effector T cells cannot traffic through. Our data support the use of B cells as a novel delivery system that can effectively engage secondary CD8+ T-cell activation using prime-boost strategies.
Materials and methods
Animals
C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA) and housed in a specific pathogen-free facility. B6.PL-Thy1a/CyJ, B6.129S2-IGH-6TM1CGN/J (referred to as “B−/− mice”), and B6.129P2-H2-Kbtm1 H2-Dbtm1 N12 (referred to as “KbDb−/− mice”) were purchased from The Jackson Laboratory (Bar Harbor, ME) and Taconic Breeding Laboratories (Germantown, NY), respectively. CD11c-DTR transgenic mice were bred in the Central Animal Facility at McMaster University. All animal studies complied with Canadian Council on Animal Care guidelines and were approved by McMaster University’s Animal Research Ethics Board.
Recombinant viruses
The E1, E3-deleted recombinant adenovirus (Ad) and M protein mutant vesicular stomatitis virus (VSV) used in this study have been described previously.20,21 Ad-GP33 and VSV-GP33 encode the dominant CD8+ and CD4+ T-cell epitopes of the lymphocytic choriomeningitis virus glycoprotein (LCMV-GP33-41 and LCMV-GP61-80, respectively). Ad-SIIN and VSV-SIIN express the dominant CD8+ T cell epitope from chicken ovalbumin (OVA257-264). Recombinant vaccinia (Vac) expressing GP33 (Vac-GP33; provided by Lindsay Whitton, The Scripps Research Institute, La Jolla, CA) or SIINFEKL (Vac-SIIN) has been described.21,22 The Ad-BHG and VSV-MT were control vectors, lacking a transgene.
Peptides
The H-2Kb-restricted OVA peptide OVA257-264; SIINFEKL was synthesized by Biomer Technologies (San Francisco, CA). The H-2Db-restricted peptide of LCMV-GP (GP33-41; KAVYNFATM) was purchased from the Dalton Chemical Laboratory (Toronto, Canada). Peptides were dissolved in distilled water and stored at −20°C.
Cell culture and viral infection
B cells were purified from the spleen of wild-type (WT) C57BL/6 mice using negative selection kits (Miltenyi Biotec, Teterow, Germany). Purified B cells were cultured with adipocyte-conditioned medium supplemented with 20 ng/mL interleukin-2, imiquimod, and Phorbol 12, 13-dibutyrate at the concentration of 2 × 106 B cells/mL, as previously described.23 Three days after culture, B cells were pulsed with VSV or Vac vectors at a multiplicity of infection (MOI) of 25 for 2 to 24 hours. Viral vector–pulsed freshly isolated B cells were used as a comparison.
The procedure for generating bone marrow–derived DCs has been described previously.24 Seven days after culture, DC infection was carried out as described previously
Supernatants from B cell or DC culture were collected at the start of and 2 hours after infection following centrifugation to remove cells. Vero cells were infected with a dilution series of these collected supernatants or purified VSV-GP33 (as positive control) in triplicate for 1 hour before overlay of 1% agarose gel onto the culture dish. Viral titers were quantified by the plaque assay after further incubation for 24 to 48 hours.
Prime-boost protocol
Mice were immunized by intramuscular injection with 1 × 108 plaque-forming units (PFU) Ad-GP33 or intraperitoneal (IP) injection with 2 × 107 pfu Vac-GP33 as a primary vaccine. Three million vector- or peptide-pulsed antigen-presenting cells (APCs) were given by IV or footpad injection as a secondary vaccine at the indicated intervals. VSV-GP33 was given by IV injection either as a primary vaccine (1 × 109 PFU) or as a control secondary vaccine (2 × 106 to 1 × 108 PFU).
Detection of antigen-specific T-cell responses
Single cell suspensions prepared from different tissues were restimulated with peptides (1 μg/mL) at 37°C for 5 hours and brefeldin A (Golgi Plug, 1 μg/mL; BD Biosciences, Mississauga, ON, Canada) was added during the last 4 hours of incubation. Cells were treated with Fc block and stained for surface expression of CD3 and CD8. Cells were subsequently fixed, permeabilized (Cytofix/Cytoperm, BD Biosciences) and stained for intracellular interferon-γ. Data were acquired using a FACSCanto with FACSDiva software (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
BrdU incorporation assay
FTY720 (4 mg/kg body weight) (Cayman Chemical, Ann Arbor, MI) was injected IP to inhibit lymphocyte egress from peripheral lymphoid organs 2 hours before secondary vaccination. An IP injection of 1 mg 5-bromo-2'-deoxyuridine (BrdU, BD Biosciences) was delivered 24 hours before tissue harvesting and mice were given drinking water containing BrdU until harvest (0.8 mg/mL). After Fc blocking, lymphocytes from different organs were stained with antibodies against CD8 and BrdU (BrdU staining kit, as per manufacturer’s instructions; BD Biosciences), and H-2Db-GP33 tetramer for antigen specific T-cell proliferation (MHC Tetramer Laboratory, Baylor College of Medicine, Houston, TX).
CFSE labeling and recovery
Cultured B cells and DCs were washed in phosphate-buffered saline and resuspended at 2 × 107 cells/mL in RPMI containing 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Sigma-Aldrich, Pakville, ON, Canada). After incubation at 37°C for 15 minutes, cells were washed thoroughly with phosphate-buffered saline before IV delivery. Spleen and lymph nodes were collected at various times and digested with collagenase (250 U/mL) for 1 hour at 37°C. Cells were stained with CD19 or CD11c antibodies and analyzed by flow cytometry to determine the number of migrating B cells or DCs.
Confocal microscopy
In addition to the flow cytometric analysis described previously, spleen and lymph node samples were also collected and made into 8-μm frozen sections for confocal microscopic imaging. After fixation with 1% paraformaldehyde and subsequent Fc blocking, slides were stained with PE-Texas Red-conjugated rat anti-murine B220 (1:800) (BD Biosciences). The prepared slides were evaluated using a confocal laser scanning microscope (LSM 510 Meta imaging system, Carl Zeiss). Excitation of fluorescent dye was at 590 nm for PE-Texas Red labeled B220.
Chimeras
To determine the role of B cells in antigen presentation following B/VSV boosting, lethally irradiated (2 × 550 Rads; 48-hour interval) WT C57BL/6 mice received 5 × 106 bone marrow from B−/− mice plus 1 × 106 bone marrow from WT (B−/− + WT) or KbDb−/− (B−/− + KbDb−/−) donors. The only difference between these two chimeras is that B cells in the latter (B−/− + KbDb−/−) are deficient for MHC class I and therefore incapable of presenting antigen to CD8+ T cells.
To determine whether endogenous DCs were required in B/VSV-induced secondary T-cell expansion, CD11c-DTR chimeric mice were similarly prepared by transferring CD11c-DTR transgenic mouse–derived bone marrow into lethally irradiated C57BL/6 recipients (CD11c→WT), which allows multiple injections of diphtheria toxin (DT) to deplete and maintain the absence of CD11c+ DCs. As controls for both chimeras, lethally irradiated WT mice were reconstituted with WT bone marrow (WT→WT). Mice were given 3 months to reconstitute their hematopoietic system (confirmed by flow cytometry) before experimentation.
Adoptive T-cell transfer
To avoid misinterpretation of results from CD11c chimeric mice in which DT may also remove some activated CD8+ T cells that express CD11c, a group of WT congenic mice (Thy1.1) were immunized with Ad-GP33 in parallel with CD11c chimeric mice (Thy1.2). On day 14, splenic CD8+ T cells were negatively selected (StemCell Technologies, Vancouver, BC, Canada) from Ad-GP33–immunized congenic mice and adoptively transferred into similarly immunized CD11c chimeric recipients before B/VSV-GP33 boosting. Five days post-B/VSV, the frequency of congenic GP33-specific T cells in the blood was determined by flow cytometry.
Statistical analysis
GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA) was used for graphing. For statistical analyses, GraphPad Prism and Minitab Statistical Software (Minitab Inc., State College, PA) were used. Student two-tailed t test, one- or two-way analysis of variance were used to analyze the immune responses and differences between means were considered significant at P ≤ .05.
Results
B cells are superior to DCs for boosting CD8+ T-cell immunity when “pulsed” with viral-vectored vaccines
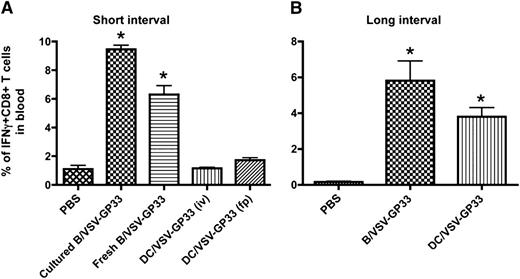
We have previously shown that DCs infected with 25 PFU per cell of recombinant VSV resulted in maximal transduction (>70%) and cells remained viable for at least 3 days.25 The same dose of VSV that expresses LCMV-GP–derived immunodominant peptide, GP33 (VSV-GP33), was used in this study to infect cultured DCs and B cells as booster vaccines. Mice were primed with a recombinant Vac expressing GP33 (Vac-GP33) for 7 to 10 days and boosted by IV injection with 3 × 106 DCs or B cells pulsed with VSV-GP33. Five days after boosting, the level of antigen-specific CD8+ T cells in the circulation was determined by intracellular staining for interferon-γ. As shown in Figure 1A, IV delivery of 3 × 106 cultured or freshly isolated B cells infected with VSV-GP33 (B/VSV-GP33) could boost a significant CTL response, whereas the same number of VSV-GP33–infected DCs (DC/VSV-GP33) failed to provoke secondary T-cell expansion, suggesting that B cells are superior to DCs for eliciting a rapid recall T-cell response. We excluded the possibility that IV injection might represent a suboptimal route for DC vaccination because footpad injection with the DCs also failed to generate a boosting effect (Figure 1A). Because cultured B cells appeared to be more potent than their fresh counterparts and could be readily expanded, we chose to use cultured B cells for all subsequent studies.
Viral vector-loaded B cells are more potent than DCs for boosting CD8+ T-cell immunity. C57BL/6 mice were primed IP with 2 × 107 pfu of Vac-GP33 and boosted with 3 × 106 B/VSV-GP33 by i.v. injection or DC/VSV-GP33 via either IV or footpad administration at a 14-day (A) or 30-day (B) interval. Five days post-boost, the frequency of GP33-specific T cells in blood were enumerated by detection of intracellular IFNγ production following in vitro stimulation with the peptide. *Significantly higher CD8+ T-cell response compared with all other groups (P < .01). Data are representative of duplicate experiments using 5 mice per group, per experiment.
Viral vector-loaded B cells are more potent than DCs for boosting CD8+ T-cell immunity. C57BL/6 mice were primed IP with 2 × 107 pfu of Vac-GP33 and boosted with 3 × 106 B/VSV-GP33 by i.v. injection or DC/VSV-GP33 via either IV or footpad administration at a 14-day (A) or 30-day (B) interval. Five days post-boost, the frequency of GP33-specific T cells in blood were enumerated by detection of intracellular IFNγ production following in vitro stimulation with the peptide. *Significantly higher CD8+ T-cell response compared with all other groups (P < .01). Data are representative of duplicate experiments using 5 mice per group, per experiment.
We next increased the interval between priming and boosting to allow for the primary immune response to wane. As expected, DC/VSV-GP33 were able to boost antigen-specific CD8+ T-cell responses on day 30 after Vac-GP33 priming, consistent with the notion that preexisting effector T cells prevent excessive antigen presentation by DCs (Figure 1B).12,15 Interestingly, B/VSV-GP33 still achieved a better boosting response compared with DC/VSV-GP33, suggesting that virus-loaded B cells are superior for generating secondary antigen-specific T-cell responses with or without the presence of preexisting CTLs.
The ability of B cells to boost secondary T-cell responses is not limited by viral vectors or target antigens
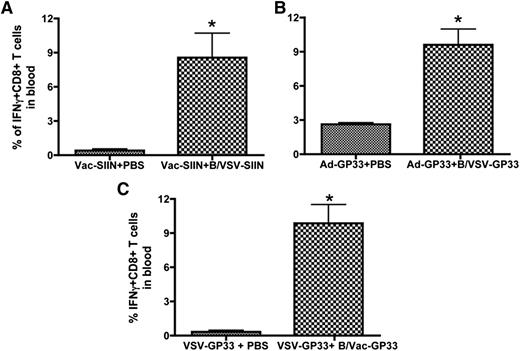
To determine whether the ability of B/VSV to boost T-cell responses was antigen dependent, we primed mice with a Vac vector expressing SIINFEKL (Vac-SIIN), a Kb-restricted immunodominant epitope from chicken ovalbumin, and followed by a boost with B cells pulsed with a recombinant VSV encoding the same epitope (VSV-SIIN). Similar to B/VSV-GP33, B/VSV-SIIN was able to trigger a robust secondary expansion shortly after Vac priming (Figure 2A), indicating that VSV-pulsed B cells can be used as an effective booster to present different antigens. We next demonstrated that VSV-pulsed B cells could mediate the booster response in the presence of higher CTL frequencies by using a recombinant Ad as the primary vaccine (Figure 2B). Finally, we investigated whether this boosting function was intrinsic to B cells or specific to VSV. In this case, we used B cells pulsed with Vac-GP33 as a booster vaccine in mice that were primed with VSV-GP33. Data in Figure 2C show that boosting with B/Vac-GP33 achieved a 10-fold increase in CTL response, reinforcing the potency and flexibility of B cells as a booster vaccine platform.
The ability of B cells to boost secondary T-cell responses is not limited by viral vectors or target antigens. C57BL/6 mice were primed with Vac-SIIN (A) or Ad-GP33 (B). Fourteen days later, mice were boosted with B/VSV-SIIN or B/VSV-GP33, respectively. The frequency of antigen-specific T cells in blood was determined by intracellular cytokine staining 5 days after boosting. (C) C57BL/6 mice were primed with VSV-GP33 and boosted with B cells pulsed with Vac-GP33 (B/Vac-GP33) 14 days after priming. GP33-specific T-cell responses were measured 5 days post-boost. *Significantly higher CD8+ T-cell response compared with the unboosted group (n = 5/group, P < .01). IFN, interferon.
The ability of B cells to boost secondary T-cell responses is not limited by viral vectors or target antigens. C57BL/6 mice were primed with Vac-SIIN (A) or Ad-GP33 (B). Fourteen days later, mice were boosted with B/VSV-SIIN or B/VSV-GP33, respectively. The frequency of antigen-specific T cells in blood was determined by intracellular cytokine staining 5 days after boosting. (C) C57BL/6 mice were primed with VSV-GP33 and boosted with B cells pulsed with Vac-GP33 (B/Vac-GP33) 14 days after priming. GP33-specific T-cell responses were measured 5 days post-boost. *Significantly higher CD8+ T-cell response compared with the unboosted group (n = 5/group, P < .01). IFN, interferon.
Virus-pulsed B cells do not present the target antigen
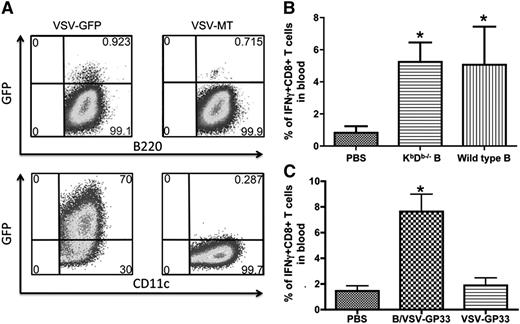
To determine whether viral infection may alter the susceptibility of B cells to CTL killing, we quantitated transgene expression in B cells and DCs following infection with VSV-green fluorescent protein. To our surprise, whereas >70% DCs were green fluorescent protein–positive, very few B cells were directly infected by the virus (Figure 3A). This observation prompted us to hypothesize that VSV-pulsed B cells may function as a carrier to deliver viral vectors instead of mediating direct antigen presentation. To address this hypothesis, we pulsed B cells from KbDb-deficient mice with VSV-GP33 and inoculated them into mice that had been primed with Vac-GP33. As shown in Figure 3B, both KbDb-deficient and WT B cells boosted secondary T-cell responses to the same level, suggesting that direct antigen presentation by VSV-pulsed B is not required for boosting of T-cell responses. These findings support the hypothesis that B cells act as virus carriers, rather than direct APCs, and are thereby capable of escaping CTL killing. Consistent with this notion, GP33 peptide-pulsed B cells failed to promote secondary CTL expansion (supplemental Figure 1A), likely because of their elimination by GP33-specific effector T cells.13,14 To provide further evidence that VSV-exposed B cells do not present antigen, we performed 2 more experiments. First, cultured B cells were pulsed with either VSV-SIINFEKL or SIINFEKL peptide directly and then stained with the 25D-1.16 antibody that recognizes the SIINFEKL/Kb complex. Second, an in vivo CTL assay was carried out in Ad-SIINFEKL–immunized mice using VSV-SIINFEKL or SIINFEKL peptide-pulsed B cells as targets. The results, summarized in supplemental Figure 1B-C, demonstrate that VSV-SIINFEKL–pulsed B cells are not bound by the 25D-1.16 antibody nor are they killed by preexisting CTL in vivo. In contrast, B cells pulsed with SIINFEKL peptide are completely bound by the 25D-I.16 antibody and completely killed in vivo.
B cells are less susceptible to VSV infection than DCs, and virus-pulsed B cells do not directly present the target antigen. (A) Cultured B cells and DCs were infected with VSV-GFP or VSV-MT at an MOI of 25. After 24 hours, GFP expression was determined in combination with surface marker staining for B220 and CD11c. (B) Vac-GP33–primed mice were boosted with WT or KbDb−/− B cells loaded with VSV-GP33, or PBS as control. The magnitude of GP33-specific T-cell responses in blood was measured by intracellular cytokine staining 5 days after boosting (n = 5/group, *P < .01 compared with PBS control). (C) Vac-GP33–primed mice were boosted with B/VSV-GP33 or 2 × 106 PFU of VSV-GP33 or PBS as control. Boosted responses in blood were measured 5 days post-secondary vaccination. *Significantly higher CD8+ T-cell response compared with VSV alone and PBS control (P < .01). Data are representative of duplicate experiments using 5 mice per group. GFP, green fluorescent protein; PBS, phosphate-buffered saline.
B cells are less susceptible to VSV infection than DCs, and virus-pulsed B cells do not directly present the target antigen. (A) Cultured B cells and DCs were infected with VSV-GFP or VSV-MT at an MOI of 25. After 24 hours, GFP expression was determined in combination with surface marker staining for B220 and CD11c. (B) Vac-GP33–primed mice were boosted with WT or KbDb−/− B cells loaded with VSV-GP33, or PBS as control. The magnitude of GP33-specific T-cell responses in blood was measured by intracellular cytokine staining 5 days after boosting (n = 5/group, *P < .01 compared with PBS control). (C) Vac-GP33–primed mice were boosted with B/VSV-GP33 or 2 × 106 PFU of VSV-GP33 or PBS as control. Boosted responses in blood were measured 5 days post-secondary vaccination. *Significantly higher CD8+ T-cell response compared with VSV alone and PBS control (P < .01). Data are representative of duplicate experiments using 5 mice per group. GFP, green fluorescent protein; PBS, phosphate-buffered saline.
To firmly establish the necessity of B cells as a carrier to deliver boosting virus, we sought to determine whether direct injection of the same amount of VSV could elicit secondary T-cell responses. At an MOI of 25, we determined that 3 × 106 B cells carried 2 × 106 PFU VSV in total after 2 hours’ infection (see “Materials and methods”). Direct injection with 2 × 106 or as high as 1 × 108 PFU (not shown) VSV-GP33 failed to boost memory T-cell responses (Figure 3C), suggesting that B cells are critically required to deliver and/or amplify VSV-mediated antigen presentation by host APCs.
To determine whether B/VSV can also boost CD8+ T-cell responses in mice that have a preexposure to VSV, we immunized mice with VSV-GP33 and then, 14 days later, boosted them with either VSV-GP33 or B cells pulsed with VSV-GP33. As expected, a minimal expansion of GP33-specific CD8+ T cells was observed by VSV-GP33 boosting, likely because of neutralizing antibodies against VSV that attenuate the boosting efficacy (supplemental Figure 2). Surprisingly, however, B/VSV-GP33 boosted a strong CTL response suggesting that a B cell–based vaccination platform may be able to bypass both preexisting CTL and neutralizing antibodies allowing for rapid, homologous prime-boosting.
Injected B cells can be recovered in the secondary lymphoid organs and are primarily localized to the follicular regions
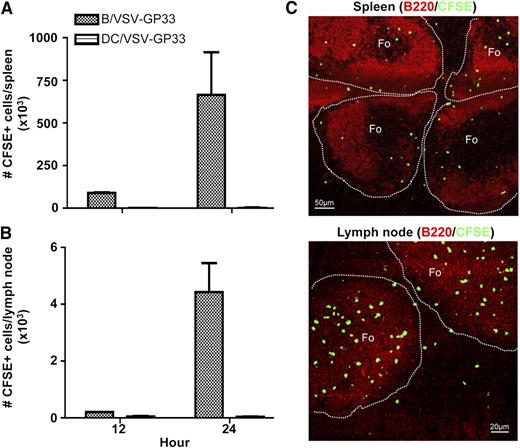
Having established a role for virus-exposed B cells as carriers, we next sought to determine whether B cells migrate to secondary lymphoid organs where central memory T cells reside. Mice were preimmunized with Ad-GP33 for 14 days to establish GP33-specific immunity before IV delivery of CFSE-labeled, VSV-GP33–exposed B cells. Figure 4A-B shows that a significant number of injected B cells could be recovered from both spleen and lymph nodes in 12 hours and reached its peak at 24 hours. In contrast, very few injected DCs could be identified in these organs.
Injected B cells can be recovered in the secondary lymphoid organs and are primarily localized to the follicular regions. C57BL/6 mice were primed with Ad-GP33 and 14 days later, mice were given IV 3 × 106 CFSE-labeled B/VSV-GP33 or DC/VSV-GP33. Spleen (A) and lymph nodes (B) were harvested at 12 and 24 hours, and the number of recovered CFSE+ cells was determined by flow cytometry (n = 3/group). This experiment was repeated twice with similar results. Frozen sections from the same treatments described previously were stained with B220 (red) and imaged using a confocal microscope. (C) A representative of these images at the 24-hour time point. Photographs were taken using a confocal laser scanning microscope (LSM 510 Meta imaging system, Carl Zeiss) with a 10× (spleen) or 20× (lymph node) objective. Fo, follicle.
Injected B cells can be recovered in the secondary lymphoid organs and are primarily localized to the follicular regions. C57BL/6 mice were primed with Ad-GP33 and 14 days later, mice were given IV 3 × 106 CFSE-labeled B/VSV-GP33 or DC/VSV-GP33. Spleen (A) and lymph nodes (B) were harvested at 12 and 24 hours, and the number of recovered CFSE+ cells was determined by flow cytometry (n = 3/group). This experiment was repeated twice with similar results. Frozen sections from the same treatments described previously were stained with B220 (red) and imaged using a confocal microscope. (C) A representative of these images at the 24-hour time point. Photographs were taken using a confocal laser scanning microscope (LSM 510 Meta imaging system, Carl Zeiss) with a 10× (spleen) or 20× (lymph node) objective. Fo, follicle.
To further visualize the localization of injected B cells within secondary lymphoid organs, spleen and lymph node sections were examined using confocal microscopy. The majority of injected B cells were located in the follicular regions (Figure 4C), suggesting that B cells may deliver viral vectors to this particular area where follicular APCs present antigens to memory T cells. This finding is consistent with the demonstration that the majority of cultured B cells possess a follicular B-cell surface phenotype (supplemental Figure 3) that may determine their homing capacity. Because effector T cells do not circulate through the marginal zone, antigen presentation by follicular APCs will not be affected by preexisting CTL.26-29
Early proliferation of antigen-specific CD8+ T cells occurs in spleen and lymph nodes requires host CD11c+ APCs for antigen presentation
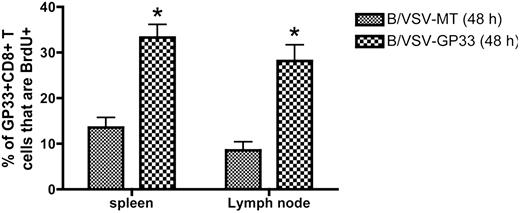
To determine that T-cell expansion indeed occurs in secondary lymphoid organs, we treated mice with FTY720 to inhibit lymphocyte egress from lymphoid organs during boosting. T-cell proliferation in the spleen and lymph notes was monitored by BrdU incorporation. Figure 5 shows that compared with the B/VSV-MT control, the earliest proliferation of GP33-specific CD8+ T cells was evident in both spleen and lymph nodes 48 hours after B/VSV-GP33 boosting.
Early proliferation of antigen-specific CD8+ T cells occurs in spleen and lymph nodes following booster vaccination with B/VSV. Ad-GP33–primed (1 × 108 PFU intramuscularly) C57BL/6 mice were boosted 14 days later with B/VSV-GP33 or B/VSV-MT as control. FTY 720 was given 2 hours before B/VSV delivery to block lymphocyte circulation. Each group received IP injection of BrdU 24 hours before tissue harvest, at which time BrdU+ GP33-specific CD8+ T cells were enumerated (n = 5/group/time point; *P < .01 compared with B/VSV-MT control). IFN, interferon.
Early proliferation of antigen-specific CD8+ T cells occurs in spleen and lymph nodes following booster vaccination with B/VSV. Ad-GP33–primed (1 × 108 PFU intramuscularly) C57BL/6 mice were boosted 14 days later with B/VSV-GP33 or B/VSV-MT as control. FTY 720 was given 2 hours before B/VSV delivery to block lymphocyte circulation. Each group received IP injection of BrdU 24 hours before tissue harvest, at which time BrdU+ GP33-specific CD8+ T cells were enumerated (n = 5/group/time point; *P < .01 compared with B/VSV-MT control). IFN, interferon.
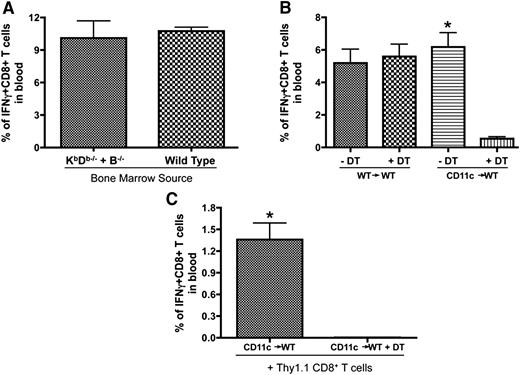
Considering the possibility that endogenous B cells and DCs are both capable of presenting the booster antigen to expand memory T cells in the follicular areas, we generated 2 chimeric strains of mice to address their role in antigen presentation (schematic of chimeric mice preparation shown in supplemental Figure 4). First, we used bone marrow cells from B−/− and KbDb−/− mice to reconstitute lethally irradiated WT mice (B−/− + KbDb−/−→WT). In these chimeras, all APC populations could express MHC class I, except B cells, which could only be derived from the KbDb−/− bone marrow. Similarly prepared recipient mice reconstituted with B−/− and WT bone marrow (B−/− + WT→WT) were included as controls (Figure 6A). The secondary expansion was not attenuated when the B cells were unable to present antigen on MHC class I (Figure 6A) demonstrating that antigen presentation by endogenous B cells was not required. The second chimeras were made using bone marrow cells from CD11c-DTR transgenic mice to reconstitute lethally irradiated WT mice (CD11c→WT). As such, multiple doses of DT could be delivered to deplete and maintain the absence of CD11c+ DCs (protocols for DT injection shown in supplemental Figure 4). Figure 6B shows that DT injection abrogated the boosting effect of B/VSV-GP33 in CD11c-DTR chimeric mice but not WT control (Figure 6B), suggesting that host DCs may mediate antigen presentation to trigger memory T-cell proliferation. To ensure that CD8+ T cells were not affected by DT treatment, we transferred antigen-primed CD8+ T cells from WT mice to CD11c-DTR chimeric mice before B/VSV boosting. DT injections abrogated expansion of transferred WT CD8+ T cells, confirming the requirement of CD11c+ DCs instead of removal of certain CD8+ T cells that may also express CD11c (Figure 6C).30,31
Endogenous DCs but not B cells are required for secondary expansion of CD8+ T cells. (A) Lethally irradiated C57BL/6 mice received 5 × 106 bone marrow cells from B−/− mice plus 1 × 106 bone marrow cells from KbDb−/− (B−/− + KbDb−/−) or WT (B−/− + WT) donors. Mice were primed with Vac-GP33 and boosted with B/VSV-GP33 after complete hematopoietic reconstitution. GP33-specific CD8+ T cells were quantified in blood at day 5 post-B/VSV boosting (n = 6/group). (B) CD11c-DTR chimeric mice (CD11c→WT) and their WT controls (WT→WT) were primed with Ad-GP33 and boosted with B/VSV-GP33. One day prior to B/VSV and every other day thereafter, mice received 100 ng of DT (IP) to deplete CD11c+ cells. GP33-specific CD8+ T cells were quantified in blood at day 5 post-VSV boosting (n = 5/group, *P < .01 compared with DT injection). (C) Negatively selected splenic CD8+ T cells from Ad-GP33–immunized congenic mice (Thy1.1) were adoptively transferred into similarly immunized CD11c chimeric recipients (Thy1.2) before B/VSV-GP33 boosting. DT injections were carried out as described previously and GP33-specific Thy1.1+CD8+ T cells were quantified in blood at day 5 post-VSV boosting (n = 5/group, *P < .01 compared with DT injection).
Endogenous DCs but not B cells are required for secondary expansion of CD8+ T cells. (A) Lethally irradiated C57BL/6 mice received 5 × 106 bone marrow cells from B−/− mice plus 1 × 106 bone marrow cells from KbDb−/− (B−/− + KbDb−/−) or WT (B−/− + WT) donors. Mice were primed with Vac-GP33 and boosted with B/VSV-GP33 after complete hematopoietic reconstitution. GP33-specific CD8+ T cells were quantified in blood at day 5 post-B/VSV boosting (n = 6/group). (B) CD11c-DTR chimeric mice (CD11c→WT) and their WT controls (WT→WT) were primed with Ad-GP33 and boosted with B/VSV-GP33. One day prior to B/VSV and every other day thereafter, mice received 100 ng of DT (IP) to deplete CD11c+ cells. GP33-specific CD8+ T cells were quantified in blood at day 5 post-VSV boosting (n = 5/group, *P < .01 compared with DT injection). (C) Negatively selected splenic CD8+ T cells from Ad-GP33–immunized congenic mice (Thy1.1) were adoptively transferred into similarly immunized CD11c chimeric recipients (Thy1.2) before B/VSV-GP33 boosting. DT injections were carried out as described previously and GP33-specific Thy1.1+CD8+ T cells were quantified in blood at day 5 post-VSV boosting (n = 5/group, *P < .01 compared with DT injection).
Discussion
Although CTL-mediated elimination of antigen-bearing migratory DCs is a necessary regulatory mechanism in preventing excessive T-cell responses, it poses a barrier for acceleration of booster immunization.12,15 Here, we report that in contrast to DCs, viral vector–loaded B cells are not affected by the presence of CTL and can effectively trigger secondary expansion of antigen-specific CD8+ T cells shortly after primary immunization. We provide evidence that B cells function as ferries in this scenario (ie, not directly infected by viral vectors) and deliver viral vectored vaccines to follicular regions of the secondary lymphoid compartment where host DCs mediate antigen presentation. Interestingly, B cells were still more potent than DCs even at the later time point when circulating effector T cells declined, suggesting that vector-pulsed B cells represent a novel boosting agent with the flexibility to both accelerate and enhance secondary CD8+ T cell responses.
We and others have previously shown that repeated immunizations using DC-based vaccines failed to increase the number of antigen-specific CD8+ T cells.13,32 Evidently, CD8+ CTL activated by primary immunization were efficient to eliminate antigen-carrying DCs in peripheral tissues, preventing access of central memory T cells to booster antigens.14,33,34 Moreover, a recent study has shown that effector T cells can reenter reactive lymph nodes and attenuate antigen presentation by killing newly arrived DCs and antigen-loaded residential DCs.35 These data clearly demonstrate that the efficacy of booster immunizations, which rely on DCs for antigen transportation, will be limited by this CTL-mediated negative feedback mechanism, especially during primary and chronic immune responses. The data presented in the current study are consistent with this notion, in which boosting with vector-infected DCs or direct injection with an equivalent dose of viral vector as was loaded onto cell carriers failed to induce CD8+ T-cell expansion. In contrast, vector-exposed B cells were not effectively infected and thus did not express the target antigen, leading to their escape from circulating CTL. The VSV glycoprotein binds to a ubiquitously expressed receptor present on virtually all nucleated cells and for this reason is commonly used to pseudotype lentiviral vectors.36 Thus the viral particles are able to bind to these B cells, but the cells do not allow for viral infection, and therefore simply serve as vehicles to deliver bound virus in vivo. Once B cells migrate to lymphoid organs, they hand off viral vectors to local DCs that process and present antigens to memory T cells. This conclusion is supported by our data that the earliest proliferation of antigen-specific T cells was observed in both spleen and lymph nodes following B cell–based booster immunization, which required the presence of host DCs.
The next question is how local DCs escaped CTL-mediated killing. Several groups have shown that CD8+ memory T cells are located in the T-cell zones of lymph nodes and spleen surrounded by follicular B cells.28,29 Particularly, in the spleen, effector CD8+ T cells are separated from memory CD8+ T cells by the marginal zone and the former cannot circulate through the follicular region. It has been shown that upon secondary exposure to lymphocytic choriomeningitis virus or Listeria monocytogenes, memory CD8+ T cells rapidly expanded and mobilized from the B-cell follicles to the red pulp via bridging channels.26,27 After the immune response subsided, memory CD8+ T cells homed back to the B-cell follicles, whereas effector cells remained in the splenic red pulp. These results point to the possibility that follicular APCs were responsible for memory CD8+ T-cell activation. Our demonstration that intravenously administered B cells were largely localized in the follicular areas, together with the requirement of host DCs, suggests that DCs within the follicles are likely the antigen presenters that can avoid CTL-mediated killing.
Interestingly, a previous study and our recent report have shown that early boosting can be achieved by delivering a sufficiently high dose of bacterial or viral vectors at the height of the primary CD8+ T-cell response when very few memory T cells are detectable.37,38 However, it is not clear whether these approaches simply load more DCs with antigen than CTL can clear or whether the high dose actually results in antigen delivery to APCs in the follicular areas. These questions are currently under investigation in our laboratory. Nevertheless, natural homing of virus-loaded B cells to the follicular region increases the efficiency of antigen delivery to a place where central memory T cells are located, rendering them a superior booster agent even in the absence of circulating CTL and a safer platform to minimize vector-associated toxicity.
Although several studies have argued that DCs can be modified to increase their resistance to CTL killing, their migration capacity remains a challenge for the development of DC-based vaccines.39-41 We and others have shown that only a small fraction (<1%) of locally inoculated DCs can migrate to the draining lymph nodes, whereas most remain at the site of immunization.13,42,43 IV-delivered DCs are also insufficient to traffic to the spleen and lymph nodes. In contrast, we demonstrate in the current study that IV-administered B cells could be efficiently recovered from spleen and all lymph nodes, suggesting that vector-pulsed B cells can deliver antigen and mediate T-cell expansion in multiple lymphoid organs.
VSV has proven to be a potent vaccine vector for immunotherapy for both cancer and infectious diseases.38,44-46 However, rapid induction of neutralizing antibodies against VSV prevents repeated administration of the virus.47 The demonstration that VSV-pulsed B cells could boost CTL responses in mice primed with VSV may offer a solution to this problem, although the underlying mechanisms remain to be determined.
Overall, in addition to the advantages of easy preparation and broad distribution in lymphoid organs upon IV delivery, we have identified another novel application of B cells as a carrier to bypass CTL killing and neutralizing antibodies, leading to increased efficiency of antigen delivery to the right location for acceleration and enhancement of booster immunization. Our findings reinforce the idea that B-cell–based cellular vaccines represent a highly promising vaccination approach, which may not only complement the use of DCs but also offer additional advantages over DC vaccines, especially for amplification of CD8+ T-cell recall responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Natasha Khazdan for producing the vesicular stomatitis virus vectors.
This work was supported by grants from the Canadian Institutes of Health Research (MOP-67066) and the Ontario Institute for Cancer Research (Y.W.).
Authorship
Contribution: L.Z., B.W.B., and Y.W. designed the study; D.S., B.D.L., and J.L.B. provided intellectual input and helped design research; L.Z., B.W.B., L.C., J.P., J.E.B., J.D.B., and A.R. did the research; and L.Z. and Y.W. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yonghong Wan, Department of Pathology and Molecular Medicine, McMaster University, Room MDCL-5024, 1200 Main St West, Hamilton, Ontario, Canada, L8N 3Z5; e-mail: wanyong@mcmaster.ca.