On page 5252 in the 31 May 2012 issue, there is an error in flow cytometry dot plots that represent F4/80+ and PDCA-1+ cells in Figure 1C. An error during the assembly of the original Figure 1C caused the exclusion of the F4/80 dot plot and a duplicate inclusion of the PDCA-1 dot plot. The gating strategy is now reported in a new Figure 1C, which has been assembled using dot plots from an independent analysis. The corrected Figure 1 is shown.
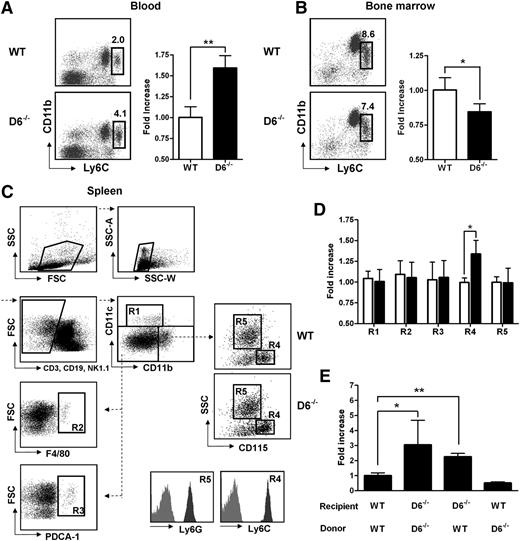
Lack of D6 on the nonhematopoietic compartments causes increased number of inflammatory monocytes. (A) Percentage in CD45+ gate (left panel) and fold increase of absolute number (right panel) of CD11b+/Ly6Chigh monocytes recovered from blood in D6–/– (black bar) versus WT mice (white bar). (B) Fold increase of percentage of CD11b+/Ly6Chigh monocytes in total BM CD45+ leukocytes of D6–/– (black bar) and WT mice (white bar). (C) Gating strategy for evaluating the percentage and absolute numbers of leukocytes in mouse spleen. Dead cells, doublets, and CD3+, CD19+, and NK1.1+ cells were excluded for further analysis. Cell labeling with anti-CD11b and CD11c allows identification of myeloid DCs (R1: CD11b+/CD11chigh), red pulp macrophages (R2: F4/80+), and plasmacytoid DCs (R3: PDCA-1+) in the CD11cdim/CD11bdim gate. Inflammatory monocytes (R4) and neutrophils (R5) are distinguished within the CD11bhigh population as SSClow/CD115+/Ly6Chigh and SSChigh/CD115–/Ly6Ghigh, respectively. Gates are based on isotype controls. (D) Fold increase of the absolute number of cells in spleen of D6–/– (black bar) versus WT (white bar) mice based on gated strategy used in panel C. (E) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6–/– (Ly5.2) mice reconstituted with WT Ly5.1 or D6–/– BM cells and in WT mice (Ly5.1) reconstituted with D6–/– BM cells versus WT mice (Ly5.1) reconstituted with WT mice (Ly5.2). In all panels, data are representative of 4 independent experiments (n = 10 mice/experiment). *P < .05. **P < .005.
Lack of D6 on the nonhematopoietic compartments causes increased number of inflammatory monocytes. (A) Percentage in CD45+ gate (left panel) and fold increase of absolute number (right panel) of CD11b+/Ly6Chigh monocytes recovered from blood in D6–/– (black bar) versus WT mice (white bar). (B) Fold increase of percentage of CD11b+/Ly6Chigh monocytes in total BM CD45+ leukocytes of D6–/– (black bar) and WT mice (white bar). (C) Gating strategy for evaluating the percentage and absolute numbers of leukocytes in mouse spleen. Dead cells, doublets, and CD3+, CD19+, and NK1.1+ cells were excluded for further analysis. Cell labeling with anti-CD11b and CD11c allows identification of myeloid DCs (R1: CD11b+/CD11chigh), red pulp macrophages (R2: F4/80+), and plasmacytoid DCs (R3: PDCA-1+) in the CD11cdim/CD11bdim gate. Inflammatory monocytes (R4) and neutrophils (R5) are distinguished within the CD11bhigh population as SSClow/CD115+/Ly6Chigh and SSChigh/CD115–/Ly6Ghigh, respectively. Gates are based on isotype controls. (D) Fold increase of the absolute number of cells in spleen of D6–/– (black bar) versus WT (white bar) mice based on gated strategy used in panel C. (E) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6–/– (Ly5.2) mice reconstituted with WT Ly5.1 or D6–/– BM cells and in WT mice (Ly5.1) reconstituted with D6–/– BM cells versus WT mice (Ly5.1) reconstituted with WT mice (Ly5.2). In all panels, data are representative of 4 independent experiments (n = 10 mice/experiment). *P < .05. **P < .005.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal