Key Points
cAMP inhibits p53 accumulation and cell death in BCP-ALL cells but not normal BCPs, providing a possible therapeutic window for intervention.
Activation of the PGE2-cAMP-PKA axis might be exploited by leukemic cells to suppress oncogene- and treatment-induced p53 activation.
Abstract
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most commonly occurring pediatric cancer. Despite its relatively good prognosis, there is a steady search for strategies to improve treatment effects and prevent the undesired side effects on normal cells. In the present paper, we demonstrate a differential effect of cyclic adenosine monophosphate (cAMP) signaling between normal BCPs and BCP-ALL blasts, pointing to a potential therapeutic window allowing for manipulation of cAMP signaling in the treatment of BCP-ALL. By studying primary cells collected from pediatric BCP-ALL patients and healthy controls, we found that cAMP profoundly decreased basal and DNA damage-induced p53 levels and cell death in malignant cells, whereas normal BCP counterparts displayed slightly augmented cell death when exposed to cAMP-increasing agents. We did not find evidence for a selection process involving generation of increased basal cAMP levels in BCP-ALL cells, but we demonstrate that paracrine signaling involving prostaglandin E2-induced cAMP generation has the potential to suppress p53 activation and cell death induction. The selective inhibitory effect of cAMP signaling on DNA damage-induced cell death in BCP-ALL cells appears to be an acquired trait associated with malignant transformation, potentially allowing the use of inhibitors of this pathway for directed killing of the malignant blasts.
Introduction
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is a malignant lymphoid neoplasm consisting of cells that morphologically and immunophenotypically resemble BCPs normally found in the bone marrow.1 The disease occurs most commonly in children with an incidence of 3-4/100 000 and accounts for ∼35% of all pediatric cancers. It is treated with combinatorial chemotherapeutic regimens with varying intensity according to risk stratification of the patients and currently has a total survival rate of 75% to 85%.1-3 Although the outcome of pediatric ALL has been greatly improved during the past 5 decades, there is an ongoing search to improve current treatment strategies. This is partly aimed at increasing treatment efficiency in diagnostic subgroups with poor prognosis and partly aimed to reduce the incidence of late effects of intensive chemotherapy such as secondary neoplasms, endocrine dysfunction, cardiac-related disease, and cognitive and psychosocial problems.4,5 Adult ALL patients generally have a poorer prognosis, with overall survival rates of 40% to 50%.6 Principles of treatment are similar as for pediatric patients but with the addition of irradiation, which is still employed against central nervous system involvement and localized infiltrates.
Suppression of normal p53 function is generally assumed to be a prerequisite for malignant cell development.7 In contrast to most carcinomas, the occurrence of p53 mutations is relatively rare in hematological malignancies. More specifically, close to all BCP-ALL cases present with wild-type p53 at initial diagnosis.8 It is therefore reasonable to assume that ALL blasts depend on alternative mechanisms to mitigate p53 function. One such mechanism is thought to be the overexpression of HDM2, the main negative regulator of p53, which is found to occur in 20% to 30% of pediatric ALL cases.9 In addition to its role in tumor development, suppression of p53 activity has been shown to contribute to treatment resistance.10 Malignancies arising from the lymphoid cell compartment generally respond relatively well to multimodal chemotherapy and irradiation, with cells typically being cleared by apoptotic cell death upon p53 activation.11-13 In spite of this, poor treatment response or relapse after initial remission occurs in a significant fraction of patients with lymphomas and lymphoid leukemias.
In the BCP-ALL cell line Reh, we previously demonstrated that augmented cyclic adenosine monophosphate (cAMP) levels can protect the cells from DNA damage-induced apoptotic cell death due to attenuation of p53 accumulation and p53-dependent signaling.14,15 cAMP is the prototypical second messenger that is generated by adenylyl cyclase (AC) upon stimulation of G protein-coupled receptors (GPCRs). In cells of the immune system, cAMP is well established as an important physiological signal transducer.16 Lymphocytes possess GPCRs for catecholamines and prostaglandin E2 (PGE2), and engagement of these receptors by their respective ligands has been shown to exert a growth-inhibitory effect mediated by the elevation of cAMP levels.17,18 ALL blasts have also been demonstrated to express functional PGE2 receptors19 ; however, the possible role of cAMP signaling in ALL physiology has yet to be determined. The purpose of the present study was to reveal a possible unique role of cAMP as a modulator of DNA damage responses in malignant vs normal BCPs and explore the possible relevance of such cAMP-mediated signaling for the pathophysiology and treatment of BCP-ALL.
Materials and methods
Reagents and antibodies
Forskolin and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO), 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphate (8-CPT-cAMP) from BioLog LSI (Hayward, CA), and PGE2 from Biosense (Bergen, Norway). 8-bromoadenosine-3′,5′-cyclic monophosphorothiorate, Rp-isomer (Rp-8-Br-cAMPS) was a kind gift from Professor Kjetil Taskén (The Biotechnology Center of Oslo, University of Oslo, Norway). Antibodies directed against p53 (DO-1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-directed antibodies were from Sigma-Aldrich, and CD10-directed antibodies (B-E3) were purchased from Abcam (Cambridge, MA). CD10 antibodies were labeled with DSB-X biotin using the DSB-X Biotin Protein Labeling Kit from Invitrogen (Carlsbad, CA) according to the manufacturer’s instructions. CD10-PE-Cy5 was obtained from BD Pharmingen (San Jose, CA), CD19-fluorescein isothiocyanate (FITC) from Miltenyi Biotech (Bergisch Gladbach, Germany), and Alexa Fluor 488-donkey anti-mouse IgG from Molecular Probes (Carlsbad, CA).
Radiation treatment
For treatment with γ-radiation, cells were exposed to a 137Cs source at a dose rate of 4.3 Gy/min using a Gammacell irradiator from MSD Nordion (Ottawa, Canada).
Collection of samples from BCP-ALL patients and healthy volunteers
BCP-ALL patients were recruited at diagnosis from the Section for Pediatric Haematology and Oncology at Oslo University Hospital. Upon consent (in accordance with the Declaration of Helsinki), 2-3 mL of bone marrow was collected for experimental purposes during routine diagnostic bone marrow aspiration in general anesthesia. All patients were treated in accordance with the Nordic Organization for Pediatric Hematology and Oncology ALL 2008 protocol, non-high risk induction (www.nopho.org), with the exception of ALL6, who was treated with RALLE Relapse protocol.20 Healthy volunteers were recruited among medical students at the University of Oslo, and 50–100 mL of bone marrow aspirate was collected in local anesthesia. The collection of bone marrow material from patients and healthy volunteers has been approved by the Regional Ethics Committee of Norway, region Sør-Øst A, and recommended by Competent Authorities. Donor and patient characteristics are outlined in Tables 1 and 2. See supplemental data for information on allocation of samples to different experiments.
Patient characteristics
| Patient characteristics . | ALL1 . | ALL2 . | ALL3 . | ALL4 . | ALL5 . | ALL6 . | ALL7 . | ALL8 . | ALL9 . | ALL10 . | ALL11 . | ALL12 . | ALL13 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 2,9 | 1,8 | 1,8 | 8 | 14,5 | 5 | 4,4 | 12,1 | 3,5 | 15,6 | 8 | 3,4 | 3,8 |
| Sex | female | female | male | female | female | female | male | female | Female | female | male | female | male |
| Relapse | no | no | no | no | no | yes | no | no | No | no | no | no | no |
| Immuno-phenotype | pre-B | common-B | common-B | pre-B | common-B | common-B | common-B | pre-B | pre-B | common-B | pre-B | pre-B | pre-B |
| Cytogenetics | t(12;21)(p13;q22) | normal karyotype | normal karyotype | hyperdiploid | t(9;22) | normal karyotype | hyperdiploid | normal karyotype | normal karyotype | t(12;21)t(5;17) | normal karyotype | t(12;21)(p13;q22) | hyperdiploid |
| Residual disease at d 15 | 2 × 10−4 | 17.3 × 10−2 | 2 × 10−3 | 15.9 × 10−2 | 42 × 10−2 | not performed | 7 × 10−4 | 5 × 10−5 | 4 × 10−3 | 30 × 10−2 | 1 × 10−4 | 1.1 × 10−2 | 4.4 × 10−3 |
| Residual disease at d 29 | no MRD | 4.2 × 10−3 | no MRD | 1.9 × 10−2 | 27 × 10−2 | no MRD | 3 × 10−4 | no MRD | no MRD | 7.3 × 10−3 | no MRD | 3 × 10−4 | 1 × 10−4 |
| Patient characteristics . | ALL1 . | ALL2 . | ALL3 . | ALL4 . | ALL5 . | ALL6 . | ALL7 . | ALL8 . | ALL9 . | ALL10 . | ALL11 . | ALL12 . | ALL13 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 2,9 | 1,8 | 1,8 | 8 | 14,5 | 5 | 4,4 | 12,1 | 3,5 | 15,6 | 8 | 3,4 | 3,8 |
| Sex | female | female | male | female | female | female | male | female | Female | female | male | female | male |
| Relapse | no | no | no | no | no | yes | no | no | No | no | no | no | no |
| Immuno-phenotype | pre-B | common-B | common-B | pre-B | common-B | common-B | common-B | pre-B | pre-B | common-B | pre-B | pre-B | pre-B |
| Cytogenetics | t(12;21)(p13;q22) | normal karyotype | normal karyotype | hyperdiploid | t(9;22) | normal karyotype | hyperdiploid | normal karyotype | normal karyotype | t(12;21)t(5;17) | normal karyotype | t(12;21)(p13;q22) | hyperdiploid |
| Residual disease at d 15 | 2 × 10−4 | 17.3 × 10−2 | 2 × 10−3 | 15.9 × 10−2 | 42 × 10−2 | not performed | 7 × 10−4 | 5 × 10−5 | 4 × 10−3 | 30 × 10−2 | 1 × 10−4 | 1.1 × 10−2 | 4.4 × 10−3 |
| Residual disease at d 29 | no MRD | 4.2 × 10−3 | no MRD | 1.9 × 10−2 | 27 × 10−2 | no MRD | 3 × 10−4 | no MRD | no MRD | 7.3 × 10−3 | no MRD | 3 × 10−4 | 1 × 10−4 |
MRD, minimal residual disease.
Control characteristics
| Control characteristics . | BM1 . | BM2 . | BM3 . | BM4 . | BM5 . | BM6 . | BM7 . | BM8 . | BM9 . | BM10 . | BM11 . | BM12 . | BM13 . | BM14 . | BM15 . | BM16 . | BM17 . | BM18 . | BM19 . | BM20 . | BM21 . | BM22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 25 | 23 | 23 | 26 | 23 | 23 | 25 | 34 | 23 | 25 | 19 | 28 | 30 | 22 | 25 | 26 | 28 | 25 | 20 | 27 | 20 | 26 |
| Sex | female | male | male | male | male | female | male | female | female | male | male | male | male | male | female | male | male | male | male | female | male | female |
| Control characteristics . | BM1 . | BM2 . | BM3 . | BM4 . | BM5 . | BM6 . | BM7 . | BM8 . | BM9 . | BM10 . | BM11 . | BM12 . | BM13 . | BM14 . | BM15 . | BM16 . | BM17 . | BM18 . | BM19 . | BM20 . | BM21 . | BM22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 25 | 23 | 23 | 26 | 23 | 23 | 25 | 34 | 23 | 25 | 19 | 28 | 30 | 22 | 25 | 26 | 28 | 25 | 20 | 27 | 20 | 26 |
| Sex | female | male | male | male | male | female | male | female | female | male | male | male | male | male | female | male | male | male | male | female | male | female |
Isolation of primary cells and cell culture
Primary BCP-ALL blasts were isolated from bone marrow aspirates as previously described.14 For isolation of normal BCPs, mononuclear cells from 50 to 100 mL of bone marrow aspirate were subjected to depletion of CD15+ cells and subsequent positive selection of CD10+ cells (see supplemental Data for comprehensive method description). Cells were cultured at 106 cells/mL in RPMI medium supplemented with 10% FBS and penicillin/streptomycin.
Measurement of cell death
For assessment of PI-positive BCB-ALL blasts and normal BCPs, cells were labeled with CD19-FITC according to the manufacturer’s instruction. Just prior to analysis, PI was added to a final concentration of 20 µg/mL. The proportion of PI-positive cells was assessed by fluorescence-activated cell sorter analysis within the FITC-positive cell fraction.
Immunoblotting and densitometric analysis
Immunoblot analysis was performed as previously described.14 For densitometric analysis, films were scanned and the intensity of protein bands quantified using the Scion Image software (Scion Corporation, Frederick, MD). Alternatively, chemiluminescence signals were detected using the G:Box with GeneSnap software, and densitometric analysis performed with GeneTools, all from Syngene (Cambridge, UK). The densitometric values for p53 and GAPDH in control cells were set to 1, and the relative change in p53 was corrected for differences in protein loading by normalizing against GAPDH levels.
Immunocytochemistry
For immunocytochemistry, cells were resuspended in Krebs/Ringer solution at a concentration of 106 cells/mL, and 500 000 cells/sample were allowed to adhere to a 9- × 9-mm poly-D-lysine–coated coverslip placed in a 1.9-cm2 well at 37°C for 15 min. Cells were fixed, permeabilized, blocked, and stained with primary antibody (DO-1) and secondary antibody (Alexa Fluor 488-donkey anti-mouse) essentially as previously described.21 The coverslips were mounted with ProLong Gold antifade reagent with 4,6 diamidino-2-phenylindole (Molecular Probes). Pictures were taken at room temperature using an AxioCam MRm camera connected to a Cell Observer microscope equipped with a 40× objective, controlled by the AxioVision software (all from Carl Zeiss, Jena, Germany). Images were prepared using PhotoShop in accordance with the instructions of Blood.
Statistical methods
SPSS 14.0.2 for Windows was used to perform statistical analysis. The paired samples t test was used to test the significance of differences in series of experiments run on cells from the same patient source, whereas the Wilcoxon signed-rank test was used to test the significance of differences observed in series of experiments run on cells from different patients.
Results
Forskolin inhibits spontaneous and ionizing radiation-induced cell death in cultured BCP-ALL blasts but not in normal BCPs
In a pilot experiment on leukemic blasts from 3 pediatric BCP-ALL patients, we previously showed that increases in cAMP levels protect the cells from cell death induced by various DNA-damaging treatments, such as ionizing radiation (IR), doxorubicin, and cyclophosphamide.14 Here, we wished to investigate whether this effect of forskolin is a general phenomenon common to both normal and transformed BCPs, or whether it is a feature specific to the malignant blasts. For this purpose, we proceeded to examine the effect of forskolin on IR-induced cell death in primary BCP-ALL blasts isolated from 11 patients and normal BCPs from 7 healthy donors.
IR is employed in treatment of BCP-ALL on very limited indications.22 Still, we chose IR as the source of DNA damage based on our observation that in contrast to cytotoxic drugs, which were found to require dose titration for each individual sample, a fixed dose of IR induced more reproducible levels of cell death between samples. Furthermore, we have previously demonstrated that cAMP exerts a similar inhibitory effect on the DNA damage response independently of whether damage is inflicted by IR or chemotherapeutic drugs commonly used to treat BCP-ALL.14 Radiation dose titration experiments using 0.5 to 10 Gy showed that 2 Gy of IR maximally induced cell death when measured 20 h after irradiation (data not shown). This amount (2 Gy) of IR also corresponds to the daily fractions administered to patients during radiation treatment of ALL. Forskolin increases cellular cAMP levels by directly activating the ACs responsible for the conversion of adenosine triphosphate to cAMP.23,24 The dose of forskolin was chosen based on the previously described dose response of forskolin on the BCP-ALL–derived cell line Reh.14
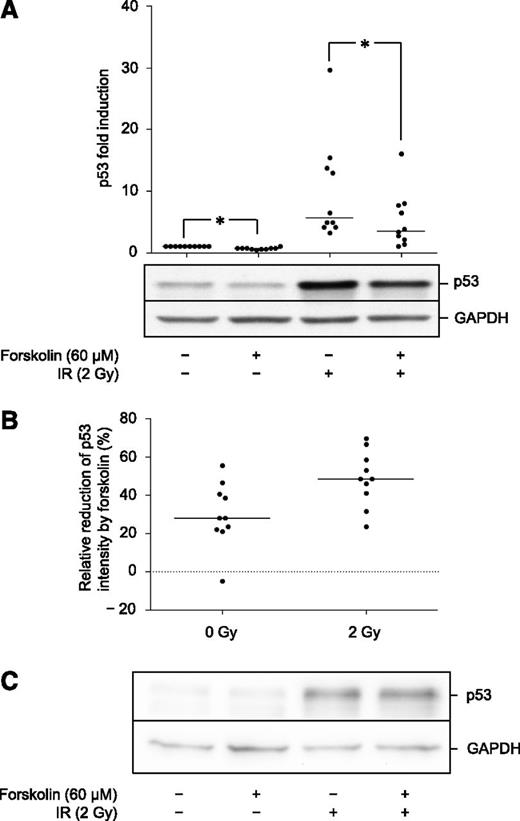
Primary BCP-ALL cells and normal BCPs were exposed to 2 Gy of IR in the absence or presence of 60 µM forskolin and examined for cell death after 20 h. To exclude contaminating cells from the cell death analysis, cells were co-stained with anti-CD19-FITC and PI, and PI staining was assessed in the CD19+ population by fluorescence-activated cell sorter analysis. As shown in Figure 1A, forskolin significantly inhibited both basal and IR-induced cell death in BCP-ALL blasts. By contrast, forskolin failed to exert a protective effect on either basal or IR-induced cell death in normal BCPs. Indeed, forskolin had a moderate but significant toxic effect in normal BCPs. This result suggests a possible shift in cell responsiveness to cAMP as a result of the malignant transformation of BCPs.
Forskolin inhibits basal and IR-induced cell death in cultured BCP-ALL blasts but not in normal BCPs. (A) Freshly isolated BCP-ALL blasts from 11 patients and normal BCPs from 7 healthy donors were cultured in the presence or absence of 60 μM forskolin for 45 min prior to treatment with 2 Gy IR as indicated. After 20 h, cell death was assessed in the CD19+ cell fraction by co-staining with FITC-labeled anti-CD19 and PI as described in Materials and Methods (n = 11 [BCP-ALL], n = 7 [normal BCPs], lines indicate median values; *P < .05 by Wilcoxon signed rank test). (B) The relative reduction in cell death by forskolin in nonirradiated and irradiated samples was calculated for each sample in Figure 1A. The resulting values are displayed in a histogram according to the cytogenetic subgroup of BCP-ALL.
Forskolin inhibits basal and IR-induced cell death in cultured BCP-ALL blasts but not in normal BCPs. (A) Freshly isolated BCP-ALL blasts from 11 patients and normal BCPs from 7 healthy donors were cultured in the presence or absence of 60 μM forskolin for 45 min prior to treatment with 2 Gy IR as indicated. After 20 h, cell death was assessed in the CD19+ cell fraction by co-staining with FITC-labeled anti-CD19 and PI as described in Materials and Methods (n = 11 [BCP-ALL], n = 7 [normal BCPs], lines indicate median values; *P < .05 by Wilcoxon signed rank test). (B) The relative reduction in cell death by forskolin in nonirradiated and irradiated samples was calculated for each sample in Figure 1A. The resulting values are displayed in a histogram according to the cytogenetic subgroup of BCP-ALL.
Although the inhibition of cell death by forskolin in BCP-ALL was statistically significant, we noted that the degree of inhibition varied greatly between samples. Figure 1B depicts the relative reduction in cell death by forskolin in nonirradiated and irradiated samples. As can be seen from this histogram, most patient samples displayed a reduction between 20% and 80%. However, 3 samples stood out with a very weak inhibitory and/or even potentiating effect on cell death. Indeed, these 3 samples responded to forskolin in a manner more similar to nontransformed B-cell precursors isolated from the bone marrow (BM) of healthy controls, and all had the similar cytogenetic characteristic of a t(12;21) translocation. This translocation results in the expression of the TEL/AML1 fusion gene, which is a common cytogenetic aberration, occurring in ∼25% of primary pediatric BCP-ALL specimens.25
Forskolin inhibits the accumulation of p53 in BCP-ALL blasts but not in normal BCPs
We have previously demonstrated that in the BCP-ALL cell line Reh, the inhibition of p53 accumulation by cAMP is required for its inhibitory effect on cell death.14 Furthermore, we showed that this effect of cAMP is mediated through augmented association of p53 with its negative regulator HDM2, resulting in increased ubiquitination and proteasomal degradation of p53.15 We have already demonstrated the proof of principle that this finding extends to 3 clinical BCP-ALL samples.14 To substantiate this finding, as well as explore the effects of cAMP on the normal BCP counterparts, blasts from 10 patients and 7 controls were exposed to IR in the absence or presence of forskolin and harvested after 4 h for detection of p53 expression by immunoblot analysis. As can be seen from Figure 2A, forskolin induced a small but significant reduction of basal p53 levels in BCP-ALL blasts. Upon exposure to IR, the blasts increased their p53 expression 5- to 30-fold. Furthermore, exposure of blasts to forskolin significantly inhibited the IR-induced expression of p53. Figure 2B shows the relative reduction of p53 intensity by forskolin for each patient sample. The median reduction in nonirradiated cells is 28% (range −5% to 56%). The median reduction in irradiated cells is 48% (range 31% to 69%).
Forskolin inhibits basal and IR-induced p53 levels in BCP-ALL blasts but not in normal BCPs. (A) BCP-ALL blasts from 10 patients were cultured in the presence or absence of 60 μM forskolin prior to IR as described in Figure 1A. At 4 h after irradiation, cells were harvested and subjected to immunoblot analysis with antibodies directed against p53 and GAPDH. The immunoblot shows the results from 1 representative patient (ALL5). Densitometric analysis of the immunoblots was performed as described in Materials and Methods. The calculated fold change in p53 intensity is presented as a scatter plot (n = 10, lines indicate median values; *P < .05 by Wilcoxon’s signed rank test). (B) The relative reduction in p53 intensity induced by forskolin on nonirradiated and irradiated cells was calculated for each sample represented in Figure 2A and presented as a scatter plot (n = 10, lines indicate median values). (C) Freshly isolated normal BCPs from 7 donors were treated with forskolin and IR as described in Figure 1A. At 4 h after irradiation, cells were harvested and flash frozen in N2 before storage at −80°C. For sufficient cell material, samples from 2 or 3 different donors were pooled for immunoblot analysis with antibodies against p53 and GAPDH. The figure shows 1 representative experiment of 3.
Forskolin inhibits basal and IR-induced p53 levels in BCP-ALL blasts but not in normal BCPs. (A) BCP-ALL blasts from 10 patients were cultured in the presence or absence of 60 μM forskolin prior to IR as described in Figure 1A. At 4 h after irradiation, cells were harvested and subjected to immunoblot analysis with antibodies directed against p53 and GAPDH. The immunoblot shows the results from 1 representative patient (ALL5). Densitometric analysis of the immunoblots was performed as described in Materials and Methods. The calculated fold change in p53 intensity is presented as a scatter plot (n = 10, lines indicate median values; *P < .05 by Wilcoxon’s signed rank test). (B) The relative reduction in p53 intensity induced by forskolin on nonirradiated and irradiated cells was calculated for each sample represented in Figure 2A and presented as a scatter plot (n = 10, lines indicate median values). (C) Freshly isolated normal BCPs from 7 donors were treated with forskolin and IR as described in Figure 1A. At 4 h after irradiation, cells were harvested and flash frozen in N2 before storage at −80°C. For sufficient cell material, samples from 2 or 3 different donors were pooled for immunoblot analysis with antibodies against p53 and GAPDH. The figure shows 1 representative experiment of 3.
The examination of p53 expression by immunoblot analysis of normal BCPs was complicated by the low number of cells obtained after isolation. However, by pooling cells from 2 or 3 donors, analyses of a total of 7 donors were performed. As can be seen from Figure 2C, p53 was induced by IR as expected, but as opposed to their leukemic counterparts, we did not find evidence that forskolin inhibited IR-induced p53 accumulation in these cells. By densitometric analysis, the median reduction by forskolin was −2% (range −6% to 12%).
The effect of forskolin on BCP-ALL blasts is mediated by cAMP signaling
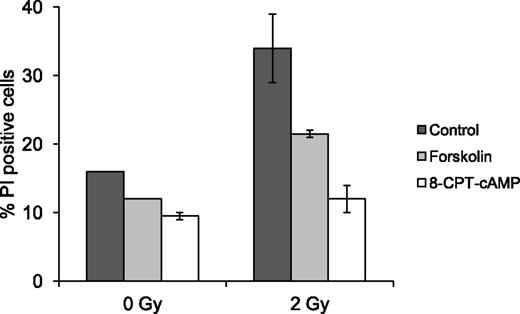
Forskolin not only activates AC and raises cAMP levels but also exerts cAMP-independent effects in certain cells.26,27 Therefore, we wished to examine whether the inhibitory effect of forskolin on IR-induced cell death in BCP-ALL blasts is indeed mediated through cAMP. To this end, we used the cell-permeable cAMP analog 8-CPT-cAMP, which upon entry into the cell directly activates cAMP effectors such as protein kinase A (PKA). The dose of 8-CPT-cAMP was chosen on the basis of dose-response experiments performed on Reh cells (data not shown). Freshly isolated BCP-ALL blasts from 2 patients were exposed to 2 Gy of IR in the absence or presence of 60 µM forskolin or 200 µM 8-CPT-cAMP and examined for cell death after 20 h. As shown in Figure 3, 8-CPT-cAMP protected the cells against both basal and IR-induced cell death even more effectively than forskolin. This supports the notion that the effect of forskolin on IR-mediated cell death in BCP-ALL blasts is mediated through stimulation of intracellular cAMP signaling. The observation that increased cAMP levels can shield transformed cells from basal and DNA damage-induced cell deaths indicate that augmented cAMP signaling can be used by BCP-ALL cells as a protective mechanism. This, together with the irresponsiveness of normal BCP cells to the cell death-inhibitory effect of cAMP, raises the possibility that expression of elevated cAMP levels can be selected for during the process of malignant transformation. To examine this notion, freshly isolated BCP-ALL blasts from 9 patients and normal BCPs from 7 voluntary donors were analyzed for their total intracellular cAMP content by cAMP enzyme immunoassay. cAMP levels in both BCP-ALL blasts and normal BCPs varied between <0.03 and 0.35 pmol/106 cells, with median values of 0.10 and 0.11 pmol/106 cells, respectively (data not shown). This result indicates that transformation of B-cell precursors is not accompanied by an increase in total cellular cAMP levels.
The effect of forskolin on BCP-ALL blasts is mediated through cAMP signaling. Freshly isolated BCP-ALL blasts from 2 patients (ALL5 and ALL9) were cultured in the presence or absence of 60 µM forskolin or 200 µM 8-CPT-cAMP for 45 min prior to treatment with IR as indicated. Cells were analyzed for cell death as in Figure 1A. The histogram shows mean values with error bars indicating the range of values.
The effect of forskolin on BCP-ALL blasts is mediated through cAMP signaling. Freshly isolated BCP-ALL blasts from 2 patients (ALL5 and ALL9) were cultured in the presence or absence of 60 µM forskolin or 200 µM 8-CPT-cAMP for 45 min prior to treatment with IR as indicated. Cells were analyzed for cell death as in Figure 1A. The histogram shows mean values with error bars indicating the range of values.
PGE2 mimics the effect of forskolin on p53 accumulation and cell death
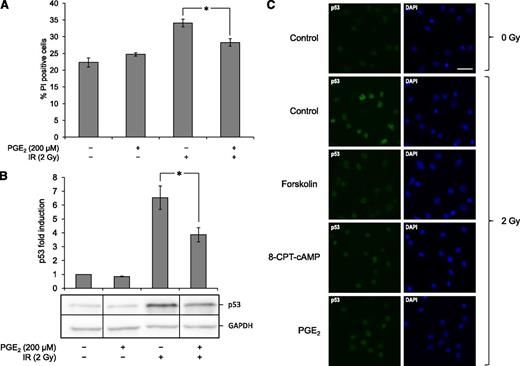
The observation that BCP-ALL cells do not possess an intrinsic ability to express elevated levels of cAMP led us to consider the possibility that extrinsic factors might augment cAMP levels in these cells. It is well established that cancerous cells do not act autonomously in the human body but engage in an intricate interplay with their surrounding stroma, which is crucial for the sustenance and progression of the disease.28 PGE2 has been implicated in promoting tumor growth in cancers of various origins and has been shown to confer protection against apoptosis.29 Furthermore, bone marrow-derived mesenchymal stem cells are known to secrete PGE2,30,31 which is relevant for BCP-development that occurs in close proximity with stroma cells in the bone marrow.32 Although thoroughly studied in many cancer types, the possible role of PGE2 signaling in ALL pathogenesis is still largely unknown; however, it has been demonstrated that ALL blasts express functional PGE2 receptors that can elicit a cAMP response upon activation.19 To examine the possible effect of PGE2 on BCP-ALL survival, primary BCP-ALL blasts were pretreated with 200 µM PGE2 for 1 h prior to irradiation with 2 Gy and analyzed for cell death after 20 h. As can be seen from Figure 4A, PGE2 conferred a significant protection against IR-induced cell death. Furthermore, by collecting cells treated as described above and analyzing for p53 expression by immunoblot analysis 4 h after irradiation, we could demonstrate that PGE2 also mimicked the effect of forskolin on p53 accumulation (Figure 4B).
PGE2 inhibits IR-induced cell death and p53 accumulation in BCP-ALL cells. (A) BCP-ALL blasts from ALL5 were cultured in the presence or absence of 200 µM PGE2 for 45 min prior to treatment with 2 Gy of IR. Subsequent cell death analysis by fluorescence-activated cell sorter 20 h after irradiation was performed as described in Figure 1A (n = 5, error bars = SEM; *P < .05 by paired samples t test). (B) A portion of cells from the experiments presented in panel 4A were harvested 4 h after irradiation, lysed, and subjected to immunoblot analysis with antibodies against p53 and GAPDH. The immunoblot shows 1 representative experiment of 5. Vertical division lines have been inserted to mark sites where the picture file has been cut and repositioned to remove lanes of intervening samples. Densitometric analyses of the immunoblots were performed as described in Materials and Methods, and calculated fold change in p53 intensity is presented as a histogram (n = 5, error bars = SEM; *P < .05 by paired samples t test). (C) BCP-ALL blasts from ALL5 were cultured in the presence or absence of 60 µM forskolin, 200 µM 8-CPT-cAMP, or 200 µM PGE2 for 45 min prior to treatment with 2 Gy of IR as indicated. At 4 h after irradiation, 500 000 cells/sample were harvested and subjected to immunocytochemistry as described in Materials and Methods.
PGE2 inhibits IR-induced cell death and p53 accumulation in BCP-ALL cells. (A) BCP-ALL blasts from ALL5 were cultured in the presence or absence of 200 µM PGE2 for 45 min prior to treatment with 2 Gy of IR. Subsequent cell death analysis by fluorescence-activated cell sorter 20 h after irradiation was performed as described in Figure 1A (n = 5, error bars = SEM; *P < .05 by paired samples t test). (B) A portion of cells from the experiments presented in panel 4A were harvested 4 h after irradiation, lysed, and subjected to immunoblot analysis with antibodies against p53 and GAPDH. The immunoblot shows 1 representative experiment of 5. Vertical division lines have been inserted to mark sites where the picture file has been cut and repositioned to remove lanes of intervening samples. Densitometric analyses of the immunoblots were performed as described in Materials and Methods, and calculated fold change in p53 intensity is presented as a histogram (n = 5, error bars = SEM; *P < .05 by paired samples t test). (C) BCP-ALL blasts from ALL5 were cultured in the presence or absence of 60 µM forskolin, 200 µM 8-CPT-cAMP, or 200 µM PGE2 for 45 min prior to treatment with 2 Gy of IR as indicated. At 4 h after irradiation, 500 000 cells/sample were harvested and subjected to immunocytochemistry as described in Materials and Methods.
To examine the effect of the various cAMP-augmenting agents on p53 expression on a single cell level, ALL blasts were treated with forskolin, 8-CPT-cAMP, or PGE2 prior to IR and examined by immunocytochemistry. Figure 4C demonstrates how p53 was generally expressed at very low levels in untreated cells, with a pronounced increase in expression upon irradiation. In the presence of forskolin, 8-CPT-cAMP, or PGE2, the radiation-induced levels of p53 were markedly diminished. In particular, there was an absence of cells displaying high intensity fluorescence, indicating that cAMP can indeed quench proper DNA damage-induced p53 accumulation in these cells.
PKA inhibition potentiates IR-induced cell death in BCP-ALL blasts but not in their normal BCP counterparts
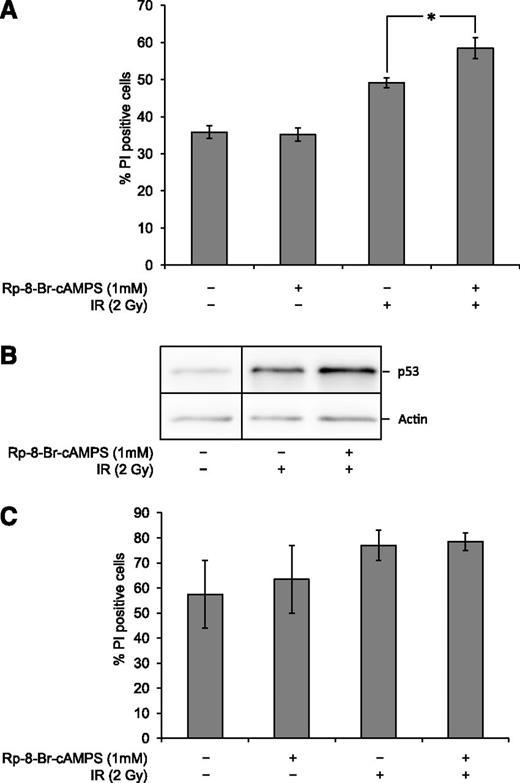
Given the observation that stimulation of cAMP signaling exerts a profound inhibitory effect on DNA damage-induced cell death in BCP-ALL blasts (Figures 1 and 3), it was important to investigate whether attenuation of cAMP signaling might enhance the cytotoxic effect of DNA-damaging agents. To do so, we took advantage of our previous finding that the effect of cAMP on p53 levels and thus cell death in the BCP-ALL cell line Reh depends on its activation of PKA.15 Consequently, we blunted the cAMP signaling pathway in BCP-ALL blasts by inhibiting the activation of PKA with Rp-8-Br-cAMPS. As shown in Figure 5A, the addition of Rp-8-Br-cAMPS to the BCP-ALL was nontoxic to nonirradiated cells but potentiated the cytotoxic effect of IR. This was accompanied by an increase in p53 levels in Rp-8-Br-cAMPS–treated cells upon irradiation (Figure 5B). Importantly, and in accordance with our data showing that augmented cAMP signaling does not inhibit cell death in normal BCPs, experiments on cells from healthy donors indicate that PKA inhibition does not potentiate IR-induced death in these cells (Figure 5C). These results imply the existence of an activated survival pathway in BCP-ALL blasts that depends on cAMP/PKA signaling.
PKA inhibition potentiates IR-induced cell death in BCP-ALL blasts. (A) Frozen aliquots of BCP-ALL blasts from ALL5 were thawed and cultured in the presence or absence of 1 mM Rp-8-Br-cAMPS 45 min prior to treatment with 2 Gy of IR with subsequent cell death analysis by fluorescence-activated cell sorter 20 h after irradiation as described in Figure 1A (n = 7, error bars = SEM; *P < .05 by paired samples t test). (B) Cells treated as in panel 5A were harvested 4 h after irradiation and subjected to immunoblot analysis using antibodies against p53 and actin. The immunoblot shows 1 representative experiment of 3. A vertical division line has been inserted to mark the site where the picture file has been cut and repositioned to remove lanes of intervening samples. (C) Freshly isolated normal BCPs from 2 donors were treated and analyzed as described in Figure 5A. The histogram shows mean values with error bars indicating the range of values.
PKA inhibition potentiates IR-induced cell death in BCP-ALL blasts. (A) Frozen aliquots of BCP-ALL blasts from ALL5 were thawed and cultured in the presence or absence of 1 mM Rp-8-Br-cAMPS 45 min prior to treatment with 2 Gy of IR with subsequent cell death analysis by fluorescence-activated cell sorter 20 h after irradiation as described in Figure 1A (n = 7, error bars = SEM; *P < .05 by paired samples t test). (B) Cells treated as in panel 5A were harvested 4 h after irradiation and subjected to immunoblot analysis using antibodies against p53 and actin. The immunoblot shows 1 representative experiment of 3. A vertical division line has been inserted to mark the site where the picture file has been cut and repositioned to remove lanes of intervening samples. (C) Freshly isolated normal BCPs from 2 donors were treated and analyzed as described in Figure 5A. The histogram shows mean values with error bars indicating the range of values.
Discussion
In the present paper, we provide evidence that the inhibitory effect of cAMP on DNA damage-induced cell death in BCP-ALL blasts appears to be an acquired trait associated with malignant transformation, as normal BCP counterparts rather exhibit increased cell death when exposed to cAMP-increasing agents. Improvement of conventional cancer therapy has 2 aims: to increase the efficiency toward the malignant cells and decrease the side effects associated with off-target effects on normal cells; in other words, to increase the selectivity of treatment effects on transformed vs normal tissues. Our demonstration that manipulation of cAMP signaling has opposing effects on BCP-ALL blasts and normal BCPs suggests the presence of a therapeutic window that would allow targeting of BCP-ALL blasts with inhibition of cAMP signaling without concomitantly affecting pro-survival pathways in normal BCPs.
It may be questioned whether the population of normal BCPs isolated in the present study constitutes a true normal counterpart of the BCP-ALL blasts. CD10+CD19+ BCPs include cells in stages from pro-B to immature B lymphocytes.33 The BCP-ALL blasts used in our study are all classified into either “common-B” or “pre-B” subtypes and thus resemble relatively early stages of normal B lymphocyte development. To avoid additional reduction in cell yield, complicating the performance of assays, we did not perform further sorting of normal BCPs to obtain a cell population representing a more limited span of B lymphocyte development. A concern regarding poor age matching of patient and healthy control groups can also be raised. The use of properly matched control groups was not appropriate in our study, because it would have involved young children as donors of considerable volumes of bone marrow. It is reassuring, however, that the donor of sample ALL5 was 15 y old and thus much closer to the control group in age than most of the other patient donors. The isolated BCP-ALL blasts from this patient responded to manipulation of cAMP signaling in a similar fashion to the blasts from the younger patients. Considering the constraints imposed by the practical and ethical considerations mentioned above, we believe that our choice of isolation methods and control population for normal BCPs resulted in a cell population representing an acceptable and relevant normal counterpart for our patient material.
The ability of cAMP to inhibit DNA damage-induced p53-dependent apoptosis in BCP-ALL cells was originally reported by our group in the cell line Reh.14,15 The findings in the present study provide a strong indication that the results obtained from Reh cells are representative for the biology of the corresponding primary BCP-ALL blasts. In addition, the primary leukemia cells displayed a pro-survival effect of cAMP on basal cell death when cultured in vitro. This is likely due to the stress imposed on the primary cells upon cultivation in an in vitro environment that both depletes these cells from protective signals normally provided by their surrounding microenvironment in vivo and exposes them to an environment known to promote oxidative stress.34 The fact that normal BCPs responded to cAMP-enhancing agents in an opposite manner to BCP-ALL blasts both with respect to in vitro culture- and radiation-induced cell death underlines the robustness of the differential findings between the normal precursors and transformed leukemic cells. Of interest, the TEL/AML1-positive subgroup of BCP-ALLs behaved more similarly to the normal counterparts with respect to cAMP-mediated effects on cell death. Although based on a very limited set of data, this points to the possibility that the observed effects of cAMP might not be linked to the development of a transformed phenotype per se but rather to alterations in intracellular signaling associated with subsets of cancer-associated genetic changes.
Due to its ability to induce apoptosis or senescence in transformed cells, normal p53 function is considered to constitute a major barrier against malignant development. As opposed to many solid tumors, wild-type p53 is almost universally retained in BCP-ALL blasts,8 and downstream defects in the p53 pathways appear to be rare in hematological malignancies.35 It is therefore reasonable to assume that wild-type p53 levels or activity must be suppressed during the development of BCP-ALL. With our observation that cAMP can indeed suppress p53 accumulation and subsequent cell death in primary BCP-ALL blasts, we hypothesized that transformation-related changes affecting cAMP-mediated signaling could be used by the malignant B-cell precursors to suppress p53 activity. In contrast to solid tumors, which have been suggested to contain elevated levels of cAMP,36,37 we did not find evidence of such a transformation-related event in BCP-ALL cells. The total cellular cAMP levels were found to vary within a similar range in BCP-ALL blasts and their normal BCP counterparts. However, it should be noted that lack of increased basal levels of cAMP in BCP-ALLs cannot completely rule out a role for the selection of constitutive cAMP signaling in transformed BCPs. It has been demonstrated that the components of the cAMP signaling pathway are highly compartmentalized in the cell, with GPCRs, ACs, cAMP effectors such as PKA and exchange protein directly activated by cAMP, and phosphodiesterases responsible for the degradation of cAMP all being brought in close proximity in distinct signalosomes within the cell.38 It is possible that aberrant activity of distinct cAMP signalosomes might contribute to localized, yet physiologically significant, elevation of cAMP levels that cannot be detected in our assay.
It is also conceivable that aberrant activation of signaling events downstream of cAMP could lead to an increase in cAMP effector functions in the absence of elevated cAMP levels. Variants of PKA are divided into 2 main classes of isozymes, PKAI and PKAII, depending on their content of the isoforms of the PKA regulatory subunit, RI and RII, respectively. Several lines of evidence indicate that PKAI activity is associated with growth and proliferation, whereas PKAII activity is associated with differentiation and decreased proliferation.39,40 Consistent with this, the RIα subunit has been found to be upregulated in a number of cell lines as well as in primary tumor cells.41,42 Furthermore, a number of human cancer tissues of different origins have been shown to express an increased RI to RII ratio.43 These observations, together with our finding that cAMP-mediated regulation of p53 expression and thus cell death depends on PKA activity,15 raise the possibility that deregulation of PKA signaling, but not cAMP levels per se, might enhance the survival of BCP-ALL blasts. Therefore, it will be interesting to examine the relative ratio of PKAI and PKAII isozymes in BCP-ALL blasts and their normal BCP counterparts.
The fact that the survival of BCP-ALL blasts is relatively poor in vitro, and that it can be improved by increasing the levels of cAMP, may be interpreted as an indication that cAMP can substitute some of the pro-survival signals normally provided to the blasts by the bone marrow microenvironment (BMME). In addition to supporting the viability and function of normal hematopoietic cells, the BMME is also assumed to provide vital support to leukemic blasts and to form niches that can confer protection against damage induced by therapeutic agents, thereby contributing to the development of treatment resistance.32,44 Given our observation that augmented cAMP signaling could increase blast survival, PGE2 was an obvious candidate to consider, as it represents a physiological paracrine substance known to be secreted by bone marrow-derived stroma cells.30,31 Our finding that PGE2 can inhibit the accumulation of the tumor suppressor p53 and subsequent DNA damage-induced cell death encourages the hypothesis that PGE2 might be an important mediator of the protective effect conferred by the BMME to the leukemic blasts. The intracellular synthesis of PGE2 is easily targeted by well-established and tolerated drugs such as nonsteroidal antiinflammatory drugs. This prompts the investigation into whether such drugs could have the potential to counteract BMME-induced therapy resistance to DNA-damaging antileukemic drugs.
There is also a possibility that the principle of attenuating cAMP signaling is already being exploited in current leukemia treatment. Glucocorticoids (GCs) play an essential part in current treatment protocols, and GC resistance is well documented as an adverse prognostic factor.45 GCs have extensive effects on cells, most of which can be traced back to the regulation of gene expression by activated GC receptors. The cytotoxic effect of GCs on leukemic cells have been well studied and seem to depend on effects on a variety of signaling pathways, such as the mitogen-activated protein kinase pathway, nuclear factor κB signaling, and glucose metabolism45 as well as more direct effects on components of the intrinsic apoptotic pathway.46 However, from studies on the antiinflammatory effects of GCs, it is also well established that GCs inhibit prostaglandin synthesis through effects on phospholipase A2 and cyclooxygenase 2.47 It is therefore an intriguing possibility that the antileukemic effects of GCs could in part be mediated through attenuation of PGE2 secretion from the BMME and thus a loss of pro-survival cAMP signaling in the leukemic blasts. This could counteract attenuation of p53 signaling and sensitize cells to apoptosis. By complementing the understanding of the complex effects of GCs on ALL blasts, our findings could open new perspectives for overcoming GC resistance in leukemia.
Our results also point to PKA as a candidate for therapeutic targeting in BCP-ALL. PKA inhibitors are not approved for clinical use at present. However, the feasibility of such treatment is currently being tested for several medical conditions, and substances such as Rp-8-Br-cAMPS have been used in preclinical experiments on animal models without apparent toxicity.48,49 Our observation that Rp-8-Br-cAMPS can indeed potentiate DNA damage-induced cell death in BCP-ALL blasts with negligible effects on normal BCPs lends promise to the further investigation into the possible clinical potential of this and similar drugs in BCP-ALL. Of particular note are the favorable results obtained on the leukemic cells from ALL5. Due to a very high yield of cells from this bone marrow sample, repeated experiments demonstrating the above-mentioned effects of PGE2 and PKA inhibition were carried out on leukemic blasts from this patient. These blasts contained the t(9;22) translocation resulting in the Philadelphia chromosome, and the presence of this chromosomal aberration with the resulting production of a BCR-ABL1 fusion protein represents an adverse prognostic feature in BCP-ALL, with 7-year overall survival in the range of 35% to 45%.50 The addition of BCR-ABL1-directed tyrosine kinase inhibitors to treatment protocols shows promise to improve the outcome for this group of patients; however, their prognosis still remains inferior to that of most other cytogenetic subgroups of BCP-ALL.50 In accordance with this, the donor of sample ALL5 unfortunately displayed poor treatment response and is the only one among our patients so far to have relapsed after induction therapy and subsequently succumbed to the disease. The clear effects of manipulating the cAMP signaling pathway in the leukemia cells from this patient is therefore of particular interest, as measures to overcome resistance to conventional therapy are long-desired for Philadelphia chromosome positive BCP-ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jannicke Holmseth for excellent technical assistance and Professor Kjetil Taskén (Biotechnology Centre of Oslo, University of Oslo, Norway) for the provision of Rp-8-Br-cAMPS and scientific advice.
This work was supported by grants from the Norwegian Research Council, the Norwegian Cancer Society, the Jahre Foundation, the Blix Family Foundation, and the Freia Foundation.
Authorship
E.H.N. designed the research, performed experiments, analyzed and interpreted data, and wrote the paper; H.K.U. performed experiments, analyzed and interpreted data, and revised the paper; P.P.D. contributed to the concept and design, collected patient material, and revised the paper; D.J. contributed to the concept and design, collected material from healthy controls and revised the paper; E.R. contributed to the concept and design, analyzed and interpreted data, and revised the paper; S.N. designed the research, analyzed and interpreted data, and wrote the paper; and H.K.B. provided the concept, designed the research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heidi Kiil Blomhoff, Department of Biochemistry, Institute of Basic Medical Sciences, University of Oslo, PO Box 1112 Blindern, N-0317 Oslo, Norway; e-mail: h.k.blomhoff@medisin.uio.no.

![Figure 1. Forskolin inhibits basal and IR-induced cell death in cultured BCP-ALL blasts but not in normal BCPs. (A) Freshly isolated BCP-ALL blasts from 11 patients and normal BCPs from 7 healthy donors were cultured in the presence or absence of 60 μM forskolin for 45 min prior to treatment with 2 Gy IR as indicated. After 20 h, cell death was assessed in the CD19+ cell fraction by co-staining with FITC-labeled anti-CD19 and PI as described in Materials and Methods (n = 11 [BCP-ALL], n = 7 [normal BCPs], lines indicate median values; *P < .05 by Wilcoxon signed rank test). (B) The relative reduction in cell death by forskolin in nonirradiated and irradiated samples was calculated for each sample in Figure 1A. The resulting values are displayed in a histogram according to the cytogenetic subgroup of BCP-ALL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/10/10.1182_blood-2012-08-452698/4/m_1805f1.jpeg?Expires=1765897979&Signature=xERb4Pv78-3EhfdQvnsOGmL8RVCRMdjG-aBymQ-a81eSBj4untekmGP-4wptLCHhQcT239w0~w-SNwmYeqYmMlsKrE2Ebk~amKTjJG91bKFkpwmweoQcLI-olkL2yswm3khDn6P7rtEyC36gkywS3wbv59JZQAn4finjZ~1DiBchZiErKjJ28x7F4uh3-nIrpXpaMX0Ava7Xb9y0e~H~F2~60wuxn80QJ2OpTFKsXlxxWpSQArzFK42hzh35QgZNAR9BGtymPt1zIblwzJUA1qSIwY9B83OBwDFYu1PQosB6f8XrqnRrbi-3fNe8OxzTpE7ciK6hBHSOAIXvSZkLLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)