In this issue of Blood, Romee et al report a novel strategy for triggering memory-like NK-cell responses with implications for NK cell–based immunotherapy.1
Although natural killer (NK) cells were identified more than 30 years ago based on their ability to kill tumor cells in the absence of prior sensitization, we have only recently begun to explore their potential in clinical therapies against cancer. Adoptive transfer of haploidentical (HLA-mismatched) NK cells to patients with cancer has resulted in complete remission in patients with treatment refractory acute myeloid leukemia (AML).2 Furthermore, 100% of children with AML remained in remission with a median follow-up time of 964 days after consolidation therapy with allogeneic NK cells.3 Together, these clinical data suggest that adoptively transferred NK cells may have a role in the treatment of selected malignancies.
By gaining further understanding of the fine specificity and molecular regulation of NK-cell functionality, we may be able to design more efficient strategies to activate, expand, and deliver NK cells for cancer immunotherapy. Recent breakthroughs in NK-cell biology that still await implementation in a clinical setting are the concepts of NK-cell education/licensing, differentiation, and the participation of NK cells in immunologic memory responses. NK-cell education refers to a dynamic functional maturation process that sets the threshold for activation in NK cells.4,5 During NK-cell differentiation NK cells undergo a number of phenotypic changes and a functional shift toward highly cytolytic cells with concomitant reduced ability to respond to cytokines.6,7 Finally, NK-cell memory refers to the surprising observation that NK cells may undergo clonal expansion and respond strongly and specifically to a re-challenge.8 Clearly, the phenotype and function of NK cells is more dynamic than previously thought and, hence, the conceptual border between innate and adaptive immunity not as clear-cut as in the scholarly literature.
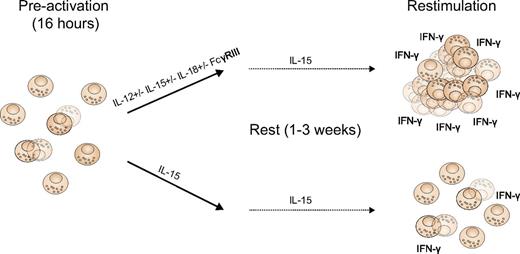
Romee et al sought to investigate whether human NK cells could “remember” earlier exposure to exogenous cytokine stimulation despite a prolonged period (weeks) of rest. Strikingly, similar to their earlier observation in mice,9 a brief pre-activation with distinct combinations of cytokines, including IL-12, IL-15, and IL-18, followed by 1 to 3 weeks of rest in low doses of IL-15, led to enhanced IFN-γ responses when re-stimulated with cytokines or cellular targets (see figure).1 Furthermore, a higher proportion of pre-activated NK cells also underwent cell division compared with controls. Although this memory-like response was not specific for any particular antigen, the enhanced IFN-γ production was inheritable and passed on to daughter cells during proliferation. Intriguingly, the heightened response to re-stimulation was confined to IFN-γ production and proliferation, and did not involve NK-cell degranulation or target cell killing. However, this is not necessarily a drawback of the current strategy, because we do not know in detail which effector function is most desirable for clinical efficacy in immunotherapeutic settings.
Cytokine-induced memory-like responses by NK cells. During the pre-activation phase, NK cells are stimulated by distinct combinations of cytokines or via the FcyRIII (anti-CD16 cross-linking) in combination with cytokines. NK cells are washed and maintained in low doses of IL-15 for up to 3 weeks. Thereafter, pre-activated cells, but not those stimulated with low dose IL-15 only, display enhanced proliferative capacity and more pronounced IFN-γ responses after restimulation with IL-12 plus IL-15, IL-12 plus IL18, or cellular targets (K562 cells).
Cytokine-induced memory-like responses by NK cells. During the pre-activation phase, NK cells are stimulated by distinct combinations of cytokines or via the FcyRIII (anti-CD16 cross-linking) in combination with cytokines. NK cells are washed and maintained in low doses of IL-15 for up to 3 weeks. Thereafter, pre-activated cells, but not those stimulated with low dose IL-15 only, display enhanced proliferative capacity and more pronounced IFN-γ responses after restimulation with IL-12 plus IL-15, IL-12 plus IL18, or cellular targets (K562 cells).
A fundamental question that remains unresolved is whether these recall responses are mediated by a true subset of memory-like NK cells or whether this phenomenon more reflects the intrinsic behavior of NK cells after cytokine stimulation. Although the cytokine pre-activated NK cells expressed surface markers associated with a less differentiated phenotype (NKG2A+KIR−CD57−), no specific marker could be used to identify the cells. However, their phenotype is clearly distinct from the highly differentiated, IFN-γ–secreting subset, expressing NKG2C and CD57 expanded in CMV-seropositive donors and after CMV reactivation in settings of transplantation.10,11 Whether there are common molecular mechanisms that can explain the propensity of both these subsets to release high levels of IFN-γ, and link these responses to those observed in mouse models of NK-cell memory, clearly warrants further investigation. For the cytokine-induced memory-like NK cells described here, the mechanism behind the heightened IFN-γ responses could be narrowed down to the translational level, because cytokine receptor-expression and downstream signaling as well as mRNA transcripts after stimulation were similar in control and pre-activated NK cells. Given the fact that the enhanced IFN-γ responses were passed on during proliferation, it is possible that epigenetic mechanisms and/or micro RNAs are involved in regulating translation of IFN-γ.
Regardless of the underlying mechanism, and the potential link to other models of adaptive NK-cell behavior, the study by Romee et al discloses an exciting new strategy to enhance the effector function of NK cells of relevance for settings of immunotherapy. The approach has conceptual resemblance with the prime-boost vaccine regimes used to trigger T-cell immunity, albeit without antigen specificity. Interestingly, triggering of FcγRIII together with cytokines also induced enhanced IFN-γ responses. Thus, treatment schedules involving repeated administration of tumor-specific antibodies, such as rituximab (anti-CD20), in combination with immunostimulatory drugs including lenalidomide and/or type I IFNs, may lead to expansion and gradually increased responsiveness of NK cells.
One challenging task facing the NK-cell research community in the years to come is to implement new knowledge on the functional dynamics and heterogeneity of NK cells in strategies of NK cell–based therapy. Taking a step in this direction, Romee et al point to a clinically relevant possibility to enhance the function of NK cells by exploiting their ability to “remember” earlier cytokine priming.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal