Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma and an aggressive malignancy. Galectin-3 (gal-3), the only antiapoptotic member of the galectin family, is overexpressed in DLBCL. While gal-3 can localize to intracellular sites, gal-3 is secreted by DLBCL cells and binds back to the cell surface in a carbohydrate-dependent manner. The major counterreceptor for gal-3 on DLBCL cells was identified as the transmembrane tyrosine phosphatase CD45. Removal of cell-surface gal-3 from CD45 with the polyvalent glycan inhibitor GCS-100 rendered DLBCL cells susceptible to chemotherapeutic agents. Binding of gal-3 to CD45 modulated tyrosine phosphatase activity; removal of endogenous cell-surface gal-3 from CD45 with GCS-100 increased phosphatase activity, while addition of exogenous gal-3 reduced phosphatase activity. Moreover, the increased susceptibility of DLBCL cells to chemotherapeutic agents after removal of gal-3 by GCS-100 required CD45 phosphatase activity. Gal-3 binding to a subset of highly glycosylated CD45 glycoforms was regulated by the C2GnT-1 glycosyltransferase, indicating that specific glycosylation of CD45 is important for regulation of gal-3–mediated signaling. These data identify a novel role for cell-surface gal-3 and CD45 in DLBCL survival and suggest novel therapeutic targets to sensitize DLBCL cells to death.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of neoplasms arising from germinal center or postgerminal center B cells.1 DLBCL is the most commonly diagnosed non-Hodgkin lymphoma2 and is an aggressive malignancy with a survival rate of ∼ 60%, partly because DLBCL cells become resistant to apoptosis induced by chemotherapy drugs.3,4 There are at least 3 molecular subtypes of DLBCL that reflect the biology of the B cell of origin.4,5 Despite the prevalence of DLBCL, and perhaps because of DLBCL heterogeneity, there is no known canonical mechanism of apoptosis resistance for the majority of DLBCL. Thus, it is critical to identify new mechanisms of apoptosis resistance in DLBCL as potential therapeutic targets.
Galectin-3 (gal-3) is expressed in ∼ 65% of primary DLBCL cases.4,6-9 Gal-3 is a member of the galectin family of immunoregulatory lectins and has both proapoptotic and antiapoptotic functions.10 Gal-3 is expressed in many types of cancer, where it has been shown to mediate apoptosis resistance.11-13 Gal-3 can localize intracellularly and participate in protein-protein interactions; for example, gal-3 has been proposed to interact with Bcl-2 protein family members at mitochondria to promote apoptosis resistance.14,15 Gal-3 can also be secreted; secreted gal-3 remains cell-surface–associated by binding to β-galactoside–containing oligosaccharides, typically on complex N-glycans and core 2 O-glycans,16 on cell-surface glycoproteins. Secreted gal-3 can form multimers via interactions of the N-terminal domain, promoting multivalent binding of the C-terminal carbohydrate recognition domain (CRD) to glycoprotein receptors. The resulting complexes have been termed galectin-glycoprotein lattices.17-19 Galectin-glycoprotein lattices modulate intracellular signaling pathways, and regulate cellular processes such as apoptosis,20 proliferation,19 and migration.21 However, while gal-3 is highly expressed in the majority of DLBCL, the role of gal-3 in apoptosis resistance in DLBCL cells has not been directly examined. In addition, if gal-3 does contribute to apoptosis resistance in DLBCL, where gal-3 acts—intracellularly or extracellularly—is also unknown.
CD45 is the major receptor tyrosine phosphatase in B cells. In normal B cells, the CD45 phosphatase regulates B-cell receptor signaling.22 CD45 is highly glycosylated, bearing both N- and O-glycans on its extracellular domain, and CD45 is a known counterreceptor for gal-3 on T cells.23,24 On T cells, gal-3 binding to CD45 modulates T-cell receptor signaling and T-cell survival. Binding of galectin-1, another galectin family member, to CD45 on T cells has also been shown to regulate phosphatase activity.20
We have found that gal-3 localizes to the cell surface of DLBCL cells, where it bound CD45 to promote apoptosis resistance. Gal-3 binding modulated CD45 phosphatase activity, and this regulation was important for apoptosis resistance. Removal of cell-surface gal-3 with GCS-100, a modified citrus pectin polysaccharide inhibitor of gal-3 that has been shown to potentiate apoptosis of other types of neoplastic cells,12,13,18,25 was sufficient to render DLBCL cells susceptible to cell death induced by different agents. This identifies a novel role for CD45 in DLBCL survival, and gal-3 as a potential therapeutic target.
Methods
Cells and reagents
Cells were maintained in RPMI 1640 with 10mM GluxaMAX, 1% MEM nonessential amino acids (Invitrogen), and 10% fetal bovine serum (Thermo Fisher Scientific).
The following reagents were used: anti–mouse gal-3 monoclonal antibody (mAb) M3/38, rat IgG2a,k isotype control, fluorescein isothiocyanate (FITC)–goat anti-rat IgG (BioLegend); anti–human CD45 mAB 2B11 + PD7/26, mouse IgG1 isotype control, FITC-goat anti–mouse IgG (DAKO); rabbit anti-Erk mAb, rabbit anti-phosphoErk1/2 (Tyr204; Cell Signaling Technology); mouse anti–human Lyn mAb H-6 (Santa Cruz Biotechnology); rabbit anti–human phosphoLyn (pY507) mAb (Epitomics), horseradish peroxidase (HRP)–goat anti–rabbit IgG, HRP-goat anti–mouse IgG (Bio-Rad); HRP-anti–rat IgG, HRP-streptavidin, FITC-streptavidin, anti–human IgM (Jackson ImmunoResearch Laboratories); potassium bisperoxo(1,10-phenanthroline)oxovanadate(V) (bp(V)phen), 1-Deoxymannojirimycin (DMNJ; Calbiochem), dexamethasone and etoposide (Sigma-Aldrich); FITC–annexin V (Invitrogen); propidium iodide (PI; BD Biosciences); Bis(sulfosuccinimidyl) suberate (BS3), 3-3′-dithiobis(sulfosuccinimidylpropionate) (DTSSP; Thermo Fisher Scientific);ImmunoPure Immobilized Protein G (Pierce); agarose-bound streptavidin, biotinylated peanut agglutinin (PNA-biotin), biotinylated Phaseolus vulgaris leukoagglutinin (PHA-L-biotin), biotinylated bovine serum albumin (BSA-biotin; Vector Laboratories); Enhanced Chemiluminescence (ECL) Detection Kit (Amersham). GCS-100 was supplied by Prospect Therapeutics; recombinant gal-3 and gal-3C were made as described.23
Immunohistochemistry
Two hundred fifty-nine cases of de novo DLBCL were obtained, sectioned, and stained with anti-gal-3 (BioLegend) as described.6,26 Slides were digitized on a ScanScope AT (Aperio Technologies) at a magnification of 20×. Images were viewed with the ImageScope viewer (Aperio Technologies) to generate detailed fields of view. Cases were scored for gal-3 expression based on staining intensity and distribution by 2 independent pathologists, who reached > 98% consensus. The study protocol for use of human tissue was approved by the University of California at Los Angeles Institutional Review Board. This study was conducted in accordance with the Declaration of Helsinki.
Cell-surface phenotyping
For gal-3 surface staining, 1 × 106 cells were transferred from flasks to FACS tubes without resuspension and fixed in 10 mg/mL DTSSP in PBS for 10 minutes, followed by 10μM Tris, pH 7.5 for 15 minutes at room temperature (RT) to cross-link soluble cell-surface gal-3 to glycoprotein receptors. Samples were washed twice in cold PBS and blocked in 1% BSA/PBS overnight. Cells were incubated with 2 μg of anti–gal-3 mAb or isotype control for 1 hour, followed by 0.4 μg of anti–rat FITC for 1 hour. CD45 staining and lectin phenotyping were performed as described,20 and on a FACScan flow cytometer (BD Biosciences).
Immunoprecipitation and Western blot
Cells were washed in PBS and lysed in NP40 lysis buffer with protease and phosphatase inhibitor cocktails (Sigma-Aldrich) as described.20 Protein concentration was determined by Bradford assay (Bio-Rad). For immunoprecipitation, lysates were diluted to 5 μg/μL with lysis buffer. Lysate (500 μg) was incubated with 30 μL of protein G beads and 2 μg of anti–gal-3 mAb or anti-CD45 mAb overnight at 4°C. Beads were washed with lysis buffer and denatured in NuPAGE sample buffer and reducing agent (Invitrogen). Where indicated, cells were fixed before lysis with BS3 for 30 minutes at RT.
RT-PCR
Death assays
Cells (2 × 105) were resuspended in medium and incubated at 37°C for 30 minutes. Cells were treated with 75 μg/mL GCS-100 for 1 hour before 10μM dexamethasone, 5 μg/mL rituximab, or 1μM etoposide for 24 hours. Cells were analyzed for annexin V binding and PI uptake as described.26 Viability was determined as % (annexin V− + PI− cells)/total live cells; cell death was determined as (100 − viability). Cells (2 × 105) were treated as above with GCS-100 and dex or rituximab before caspase-8 analysis with the Vybrant FAM Caspase-8 Assay Kit (Invitrogen) according to the manufacturer's protocol.
Inhibition of glycosylation
To inhibit production of complex N-glycans, 1 × 107 cells were treated with 2mM DMNJ for 72 hours. To inhibit production of core 2 O-glycans, 7.5 × 106 cells were transfected with 2μM C2GnT-1 siRNA or control siRNA (Santa Cruz Biotechnology) using Amaxa Nucleofector II with the human B-cell kit and program O-017 according to the manufacturer's instructions.
Phosphatase assay
Phosphatase assays were performed as described20 with the following modifications: 5 × 106 cells were resuspended in 250 μL of medium with 5μM gal-3, 5μM gal-3C, or 75 μg/mL GCS-100, as indicated.
Results
DLBCL cell expression and cell-surface localization of gal-3
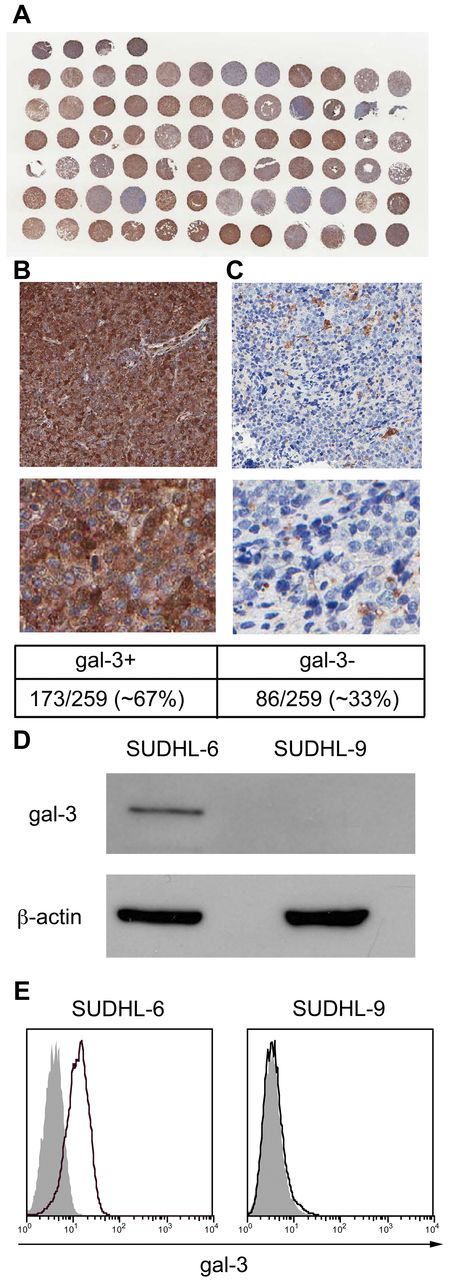
Previous studies using small cohorts (n = 10-30) have suggested that gal-3 is expressed by the majority of primary DLBCL.4,6-9 In the current study, we examined a large cohort of 259 primary DLBCL for gal-3 protein expression. A tissue microarray was stained with anti-gal-3, and bound antibody detected with HRP. A low-power magnification of a portion of the tissue array is shown in Figure 1A. Tumors were scored for gal-3 protein expression, as described in “Methods”; representative gal-3+ and gal-3− DLBCL are shown in Figure 1B and C, respectively. We found that 173 (67%) of 259 of the DLBCL samples were positive for gal-3, similar to our previous study in which 21 (66%) of 32 expressed gal-3.6
DLBCL cells express and secrete gal-3. (A-C) Tissue array of 259 primary DLBCL tissue sections was analyzed for gal-3 expression by immunohistochemistry. (A) Low-power magnification of a portion of the tissue array stained for gal-3. (B) DLBCL tissues that showed cytoplasmic staining of tumor cells were scored as gal-3+. (C) Samples in which stromal cells expressed gal-3 but no gal-3 expression was detected in tumor cells were scored as gal-3−. SUDHL-6 and SUDHL-9 cell lines derived from patients with DLBCL were analyzed for total gal-3 expression by immunoblot (D) and gal-3 cell-surface expression by flow cytometry (E). For panel E, cells were fixed before staining to retain cell-surface gal-3.
DLBCL cells express and secrete gal-3. (A-C) Tissue array of 259 primary DLBCL tissue sections was analyzed for gal-3 expression by immunohistochemistry. (A) Low-power magnification of a portion of the tissue array stained for gal-3. (B) DLBCL tissues that showed cytoplasmic staining of tumor cells were scored as gal-3+. (C) Samples in which stromal cells expressed gal-3 but no gal-3 expression was detected in tumor cells were scored as gal-3−. SUDHL-6 and SUDHL-9 cell lines derived from patients with DLBCL were analyzed for total gal-3 expression by immunoblot (D) and gal-3 cell-surface expression by flow cytometry (E). For panel E, cells were fixed before staining to retain cell-surface gal-3.
To explore the function of gal-3 in DLBCL cells, we profiled cell lines derived from DLBCL patients for gal-3 expression (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Approximately two-thirds of the cell lines examined expressed gal-3, similar to what we observed for primary tumors.6 We focused on 2 DLBCL lines: SUDHL-6 that expresses gal-3 and SUDHL-9 that does not (Figure 1D).
Gal-3 can localize to several subcellular locations, including the nucleus, mitochondria, and inner face of the plasma membrane, as well as to the cell surface.10 Gal-3 lacks a transmembrane domain, and secreted gal-3 remains cell-surface–associated by binding to cell-surface glycoproteins via the CRD. To determine whether gal-3 was present on the surface of SUDHL-6 cells, cells were fixed and analyzed by flow cytometry. We detected abundant gal-3 on SUDHL-6 cells (Figure 1E), while, as expected, no gal-3 was detected on SUDHL-9 cells.
GCS-100 removes cell-surface gal-3 from DLBCL cells
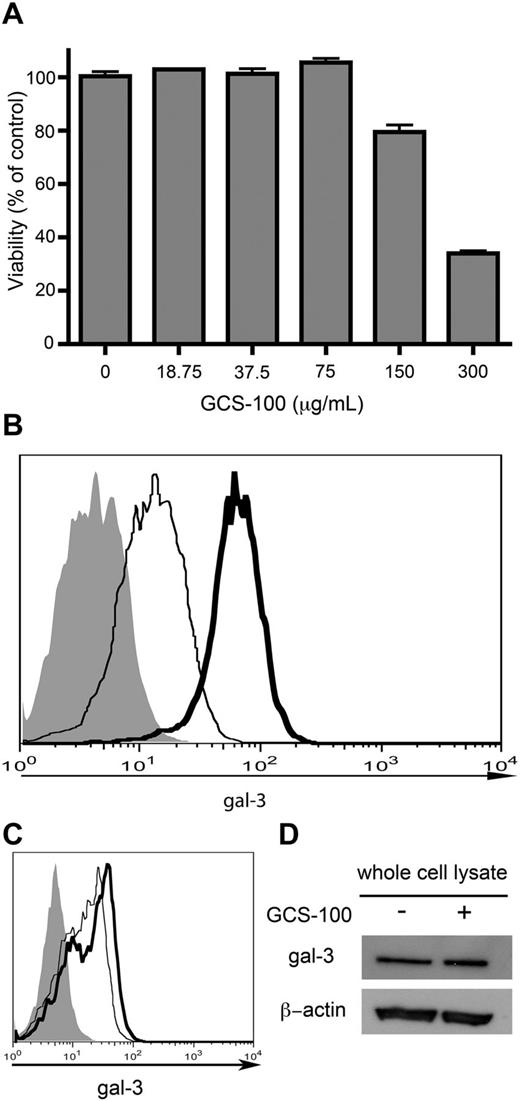
Gal-3 at different subcellular locations has been proposed to have different functions. While our previous work indicated that gal-3 in DLBCL cells had antiapoptotic activity,6 that study did not identify the subcellular site at which gal-3 was acting. Although several groups have suggested that intracellular gal-3 interacting with Bcl-2 at mitochondria has antiapoptotic activity,14 the role of cell-surface gal-3 in tumor cell survival has not been examined. Thus, we used a gal-3–specific glycan inhibitor, GCS-100, to remove cell-surface gal-3 from SUDHL-6 cells. In prior studies using GCS-100 as a gal-3 antagonist, GCS-100 at high concentrations (> 100 μg/mL) was cytotoxic for multiple myeloma cells.12,13 However, we found that at concentrations < 75 μg/mL, GCS-100 did not affect viability of SUDHL-6 cells (Figure 2A). Thus, to remove cell-surface gal-3, we treated SUDHL-6 cells with 75 μg/mL GCS-100 for 24 hours, fixed the cells, and measured cell-surface gal-3 by flow cytometry. GCS-100 typically removed ∼ 75% of cell-surface gal-3 from SUDHL-6 cells (Figure 2B). The effect of GCS-100 was reversible. When SUDHL-6 cells treated with GCS-100 were washed, we observed increased cell-surface gal-3 beginning by 1 hour compared with cells that remained in GCS-100 (Figure 2C), indicating repopulation of cell-surface gal-3 in the absence of continuous exposure to GCS-100. Importantly, we and others determined that GCS-100 did not affect galectin-1 binding to SUDHL-6 cells (data not shown),18 indicating that GCS-100 was specific for gal-3.
GCS-100 removes cell-surface gal-3 from DLBCL cells. (A) Cytotoxicity of GCS-100. Viability of SUDHL-6 cells treated with GCS-100 for 24 hours over the indicated dose range was determined. At ≤ 75 μg/mL, GCS-100 showed no toxicity, while cell death to GCS-100 alone was observed at > 75 μg/mL. Values are means ± SD of triplicates from 1 of 3 independent experiments. (B) GCS-100 removes cell-surface gal-3. SUDHL-6 cells were treated with 75 μg/mL GCS-100 (thin line) or buffer control (thick line) for 24 hours and cell-surface gal-3 measured by flow cytometry. (C) Cell-surface gal-3 is repopulated by 1 hour after removal of GCS-100. SUDHL-6 cells were treated with 75 μg/mL GCS-100 for 24 hours. GCS-100 was washed out with PBS, and cells were treated with either 75 μg/mL GCS-100 or control for 1 hour at 37°C. Cells were fixed and cell-surface gal-3 was evaluated by flow cytometry. Cells that remained in GCS-100 (thin line) had less cell-surface gal-3 compared with cells that were washed out of GCS-100 (thick line). (D) Total gal-3 was examined by immunoblot after treatment of SUDHL-6 cells with 75 μg/mL GCS-100 or buffer alone. GCS-100 treatment did not appreciably alter the total amount of gal-3 in SUDHL-6 cells.
GCS-100 removes cell-surface gal-3 from DLBCL cells. (A) Cytotoxicity of GCS-100. Viability of SUDHL-6 cells treated with GCS-100 for 24 hours over the indicated dose range was determined. At ≤ 75 μg/mL, GCS-100 showed no toxicity, while cell death to GCS-100 alone was observed at > 75 μg/mL. Values are means ± SD of triplicates from 1 of 3 independent experiments. (B) GCS-100 removes cell-surface gal-3. SUDHL-6 cells were treated with 75 μg/mL GCS-100 (thin line) or buffer control (thick line) for 24 hours and cell-surface gal-3 measured by flow cytometry. (C) Cell-surface gal-3 is repopulated by 1 hour after removal of GCS-100. SUDHL-6 cells were treated with 75 μg/mL GCS-100 for 24 hours. GCS-100 was washed out with PBS, and cells were treated with either 75 μg/mL GCS-100 or control for 1 hour at 37°C. Cells were fixed and cell-surface gal-3 was evaluated by flow cytometry. Cells that remained in GCS-100 (thin line) had less cell-surface gal-3 compared with cells that were washed out of GCS-100 (thick line). (D) Total gal-3 was examined by immunoblot after treatment of SUDHL-6 cells with 75 μg/mL GCS-100 or buffer alone. GCS-100 treatment did not appreciably alter the total amount of gal-3 in SUDHL-6 cells.
The effect of GCS-100 appeared to be limited to cell-surface gal-3. When SUDHL-6 cells treated with GCS-100 were analyzed by immunoblot to detect total gal-3 (Figure 2D), there was no appreciable difference in total gal-3 in cells treated with GCS-100 compared with control cells, indicating that GCS-100 specifically removed cell-surface gal-3 without significantly affecting the cytoplasmic gal-3 pool. This also implies that cell-surface gal-3 is a relatively small fraction of the total gal-3 in SUDHL-6 cells, as there was no appreciable difference in total gal-3 detected by immunoblot compared with the decreased cell-surface gal-3 detected by flow cytometry.
Removal of cell-surface gal-3 sensitizes DLBCL cells to apoptosis
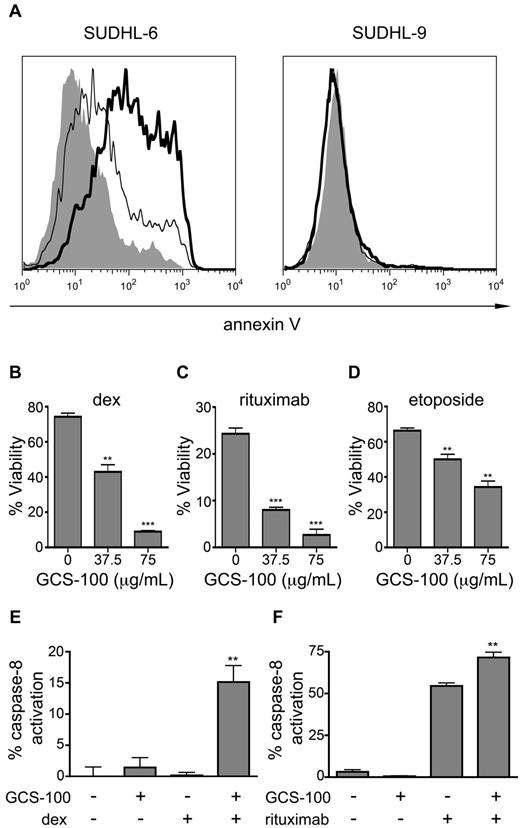
Gal-3 has been reported to have antiapoptotic activity in several types of tumor cells, including DLBCL cells6-8 ; however, removal of cell-surface gal-3 alone with 75 μg/mL GCS-100 did not decrease cell viability (Figure 2A). Thus, we asked whether removal of cell-surface gal-3 would sensitize DLBCL cells to apoptosis induced by other agents. Both SUDHL-6 and SUDHL-9 are relatively resistant to dexamethasone (dex), and dex treatment alone had minimal effect on cell viability (Figure 3A). However, removal of cell-surface gal-3 from gal-3+ SUDHL-6 cells with GCS-100 before exposure to dex resulted in an ∼ 2-fold increase in apoptosis compared with treatment with dex alone. In contrast, GCS-100 treatment of the gal-3− SUDHL-9 cells did not sensitize the cells to dex. We treated additional gal-3− and gal-3+ DLBCL cell lines with and without GCS-100 in combination with dex and observed similar results (supplemental Figure 1). Importantly, the effect of GCS-100 required cell-surface gal-3, as the BCBL1 DLBCL cell line that expressed intracellular but not cell-surface gal-3 was not sensitized to dex by treatment with GCS-100 (supplemental Figure 1).
Removal of cell-surface gal-3 with GCS-100 sensitizes DLBCL cells to cell death. (A) To remove cell-surface gal-3, SUDHL-6 and SUDHL-9 cells were treated with 75 μg/mL GCS-100 or buffer alone for 1 hour before addition of dexamethasone (dex) and analyzed for annexin V staining 24 hours later. SUDHL-6 cells were relatively resistant to dex alone (left panel, thin line) compared with control treatment (left panel, gray); however, treatment with GCS-100 before dex increased the fraction of annexin V–positive cells (left panel, thick line). SUDHL-9 cells were resistant to dex alone (right panel, thin line) compared with control treatment (right panel, gray) and no increase in annexin V binding was observed after treatment with GCS-100 plus dex (right panel, thick line). (B-D) SUDHL-6 cells were treated with increasing doses of GCS-100 for 1 hour before treatment with (B) 10μM dex, (C) 5 μg/mL rituximab, or (D) 1μM etoposide for 24 hours. Cells were analyzed for annexin V binding and PI uptake. Viability was determined as described in “Methods”; **P < .001, ***P < .0001. (E-F) Removal of cell-surface gal-3 with GCS-100 permitted caspase-8 activation. Caspase-8 activation was determined in SUDHL-6 cells treated with GCS-100 or buffer control and (E) dex or (F) rituximab; **P < .001. In panels B through F, values are mean ± SD of triplicate samples from 1 representative of 3 independent replicate experiments.
Removal of cell-surface gal-3 with GCS-100 sensitizes DLBCL cells to cell death. (A) To remove cell-surface gal-3, SUDHL-6 and SUDHL-9 cells were treated with 75 μg/mL GCS-100 or buffer alone for 1 hour before addition of dexamethasone (dex) and analyzed for annexin V staining 24 hours later. SUDHL-6 cells were relatively resistant to dex alone (left panel, thin line) compared with control treatment (left panel, gray); however, treatment with GCS-100 before dex increased the fraction of annexin V–positive cells (left panel, thick line). SUDHL-9 cells were resistant to dex alone (right panel, thin line) compared with control treatment (right panel, gray) and no increase in annexin V binding was observed after treatment with GCS-100 plus dex (right panel, thick line). (B-D) SUDHL-6 cells were treated with increasing doses of GCS-100 for 1 hour before treatment with (B) 10μM dex, (C) 5 μg/mL rituximab, or (D) 1μM etoposide for 24 hours. Cells were analyzed for annexin V binding and PI uptake. Viability was determined as described in “Methods”; **P < .001, ***P < .0001. (E-F) Removal of cell-surface gal-3 with GCS-100 permitted caspase-8 activation. Caspase-8 activation was determined in SUDHL-6 cells treated with GCS-100 or buffer control and (E) dex or (F) rituximab; **P < .001. In panels B through F, values are mean ± SD of triplicate samples from 1 representative of 3 independent replicate experiments.
These results indicated that cell-surface gal-3 confers resistance to dex, so we asked whether removing cell-surface gal-3 would sensitize SUDHL-6 cells to other apoptosis-inducing agents. SUDHL-6 cells were treated with GCS-100 in combination with dex, etoposide, or rituximab. Removal of cell-surface gal-3 with GCS-100 sensitized the cells to all 3 agents in a dose-dependent manner (Figure 3B-D). Thus, removal of cell-surface gal-3 is sufficient to sensitize gal-3+ DLBCL cells to different apoptotic stimuli.
To confirm that intracellular apoptotic pathways were being triggered, we examined activation of caspase-8, which has been shown to be activated in dex-, rituximab-, and etoposide-induced apoptosis.13,29-31 GCS-100 treatment alone did not activate caspase-8 (Figure 3E-F). However, GCS-100 in combination with dex (Figure 3E) or rituximab (Figure 3F) significantly increased caspase-8 activation compared with dex or rituximab alone. Thus, removal of cell-surface gal-3 sensitized these cells to apoptosis induced by different agents.
CD45 is the primary glycoprotein receptor for cell-surface gal-3 on DLBCL cells
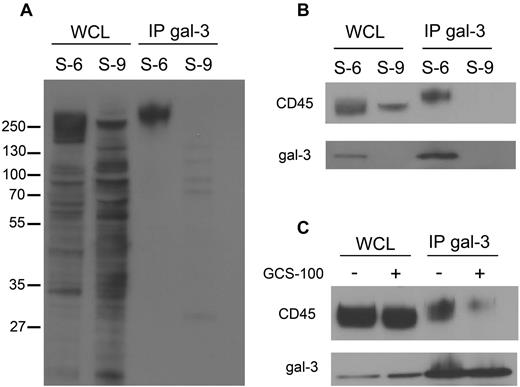
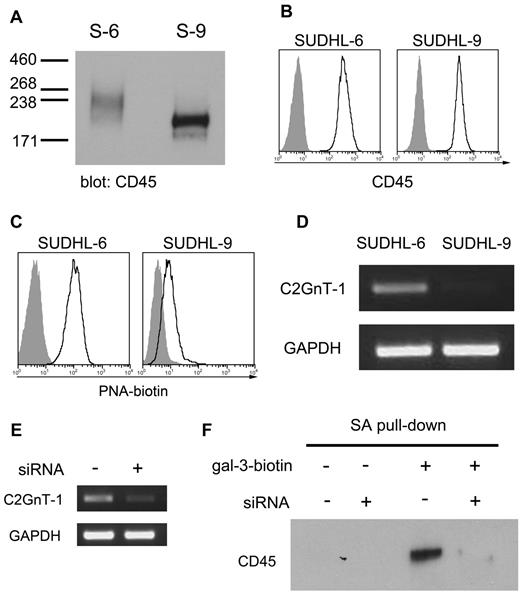
Secreted gal-3 binds to specific glycan-bearing receptors on the plasma membrane.23 To identify glycoprotein counterreceptors that could bind cell-surface gal-3, we used a coimmunoprecipitation approach that we previously used to identify galectin-1 receptors on dendritic cells.32 Cell-surface proteins on SUDHL-6 and SUDHL-9 cells were biotinylated, endogenous gal-3 was immunoprecipitated from cell extracts, and gal-3–associated cell-surface proteins were detected with streptavidin-HRP (Figure 4A). As expected, no biotinylated proteins were detected in the precipitates from gal-3− SUDHL-9 cells. In contrast, we detected 1 predominant biotinylated cell-surface glycoprotein associated with gal-3 in SUDHL-6 cells, with a molecular weight of > 250 kDa (Figure 4A).
Cell-surface gal-3 binds to CD45 on DLBCL cells. (A) To identify glycoprotein receptors for gal-3, endogenous gal-3 was immunoprecipitated from SUDHL-6 (S-6) and SUDHL-9 (S-9) cells that were biotinylated to label cell-surface glycoproteins. Precipitates were separated by SDS-PAGE and blotted to nitrocellulose and biotinylated gal-3–binding proteins detected with streptavidin-HRP. As expected, no gal-3–associated proteins were precipitated from SUDHL-9 cells. In SUDHL-6 cells, a single major gal-3–binding protein was detected at ∼ 250 kDa. (B) Immunoblotting with anti-CD45 of material immunoprecipitated in panel A confirmed that gal-3 precipitated CD45 from SUDHL-6 cells. (C) GCS-100 reduced the amount of CD45 associated with gal-3. SUDHL-6 cells were treated with GCS-100 or buffer control for 1 hour, endogenous gal-3 immunoprecipitated, and the amount of associated CD45 determined by immunoblot. WCL indicates whole-cell lysate; and IP, immunoprecipitate.
Cell-surface gal-3 binds to CD45 on DLBCL cells. (A) To identify glycoprotein receptors for gal-3, endogenous gal-3 was immunoprecipitated from SUDHL-6 (S-6) and SUDHL-9 (S-9) cells that were biotinylated to label cell-surface glycoproteins. Precipitates were separated by SDS-PAGE and blotted to nitrocellulose and biotinylated gal-3–binding proteins detected with streptavidin-HRP. As expected, no gal-3–associated proteins were precipitated from SUDHL-9 cells. In SUDHL-6 cells, a single major gal-3–binding protein was detected at ∼ 250 kDa. (B) Immunoblotting with anti-CD45 of material immunoprecipitated in panel A confirmed that gal-3 precipitated CD45 from SUDHL-6 cells. (C) GCS-100 reduced the amount of CD45 associated with gal-3. SUDHL-6 cells were treated with GCS-100 or buffer control for 1 hour, endogenous gal-3 immunoprecipitated, and the amount of associated CD45 determined by immunoblot. WCL indicates whole-cell lysate; and IP, immunoprecipitate.
Given the size and heterogeneity of the precipitated gal-3–binding glycoprotein, we considered CD45 a likely candidate, as CD45 is a receptor for gal-3 on T cells.23,24 Immunoblotting of precipitated material confirmed that the binding partner for gal-3 on DLBCL cells was CD45 (Figure 4B). Importantly, while CD45 is a heterogeneous population of glycoproteins because of differential isoform expression and differential glycosylation, we observed that only a high-molecular-weight subset of CD45 immunoprecipitated with gal-3 (Figure 4B). These results suggested that gal-3 selectively binds to a subset of CD45 on these cells.
We confirmed that gal-3 bound CD45 in a glycan-dependent manner and that gal-3 was removed from CD45 by GCS-100. SUDHL-6 cells were treated with or without GCS-100 and endogenous gal-3 was immunoprecipitated from cell extracts. Treatment with GCS-100 dramatically decreased the amount of CD45 coprecipitated with gal-3 (Figure 4C). Thus, gal-3 binds to glycans on a subset of CD45 molecules, and can be removed from CD45 with GCS-100.
Specific O-glycans on CD45 bind gal-3 to regulate susceptibility to apoptosis
As described in the previous section, CD45 is heterogeneous (Figures 4B, 5A), and gal-3 binds to a subset of high-molecular-weight CD45 on DLBCL cells (Figure 4B). Heterogeneity could result from different expression of CD45 isoforms, as well as from differential glycosylation. SUDHL-6 and SUDHL-9 cells express virtually identical amounts of total cell-surface CD45, as detected by flow cytometry using an antibody cocktail that recognizes all CD45 isoforms (Figure 5B). We examined differential CD45 isoform expression between the 2 cell lines by RT-PCR, and found that both SUDHL-6 and SUDHL-9 express similar CD45 splice variants (supplemental Figure 2A). Because SUDHL-6 and SUDHL-9 express similar amounts of CD45 and similar isoforms of CD45, we reasoned that the differences in CD45 mobility between SUDHL-6 and SUDHL-9 cells, as well as the preferential binding of gal-3 to a high-molecular-weight subset of CD45 on SUDHL-6 cells, could be because of differential glycosylation. In fact, while exogenous gal-3 added to SUDHL-9 cells bound to the cell surface as determined by flow cytometry (supplemental Figure 3A), exogenous gal-3 did not bind to CD45 on these cells (supplemental Figure 3B), further implying that gal-3 binds to a specifically glycosylated subset of CD45 expressed by SUDHL-6 cells, but not by SUDHL-9 cells.
Core 2 O-glycans on differentially glycosylated CD45 contribute to gal-3 binding. (A) SUDHL-6 (S-6) and SUDLH-9 (S-9) cells express different populations of CD45. Cell lysates were separated on a 3%-8% Tris-acetate SDS-PAGE gel to resolve high-molecular-weight isoforms of CD45, detected by immunoblot. (B) SUDHL-6 and SUDHL-9 cells express equivalent amounts of total CD45 on the cell surface. Cells were analyzed by flow cytometry using the 2B11 + PD7/26 mAbs that recognizes all 5 CD45 isoforms. (C) The abundance of asialo-core 1 O-glycans on the surface of SUDHL-6 and SUDHL-9 cells was determined by binding of biotinylated PNA, detected with avidin-FITC. (D) SUDHL-6 but not SUDHL-9 cells express mRNA encoding the enzyme C2GnT-1, that initiates addition of lactosamine chains to asialo-core 1 O-glycans. C2GnT-1 mRNA detected by RT-PCR. (E) siRNA targeting (+) reduced C2GnT-1 mRNA in SUDHL-6 cells. Nontargeted siRNA (−) was used as a control. Knockdown efficiency was determined 24 hours posttransfection by RT-PCR. (F) Reduction of C2GnT-1 expression by siRNA reduced cell-surface gal-3 association with CD45. Biotin-gal-3 or biotin-BSA was added to SUDHL-6 cells treated with siRNA for C2GnT-1 (+) or control (−). Biotinylated gal-3 and associated binding partners were precipitated from lysates with streptavidin-agarose (SA). While gal-3 precipitated CD45 from control cells, there was a significant reduction in CD45 association with gal-3 in cells with reduced C2GnT-1.
Core 2 O-glycans on differentially glycosylated CD45 contribute to gal-3 binding. (A) SUDHL-6 (S-6) and SUDLH-9 (S-9) cells express different populations of CD45. Cell lysates were separated on a 3%-8% Tris-acetate SDS-PAGE gel to resolve high-molecular-weight isoforms of CD45, detected by immunoblot. (B) SUDHL-6 and SUDHL-9 cells express equivalent amounts of total CD45 on the cell surface. Cells were analyzed by flow cytometry using the 2B11 + PD7/26 mAbs that recognizes all 5 CD45 isoforms. (C) The abundance of asialo-core 1 O-glycans on the surface of SUDHL-6 and SUDHL-9 cells was determined by binding of biotinylated PNA, detected with avidin-FITC. (D) SUDHL-6 but not SUDHL-9 cells express mRNA encoding the enzyme C2GnT-1, that initiates addition of lactosamine chains to asialo-core 1 O-glycans. C2GnT-1 mRNA detected by RT-PCR. (E) siRNA targeting (+) reduced C2GnT-1 mRNA in SUDHL-6 cells. Nontargeted siRNA (−) was used as a control. Knockdown efficiency was determined 24 hours posttransfection by RT-PCR. (F) Reduction of C2GnT-1 expression by siRNA reduced cell-surface gal-3 association with CD45. Biotin-gal-3 or biotin-BSA was added to SUDHL-6 cells treated with siRNA for C2GnT-1 (+) or control (−). Biotinylated gal-3 and associated binding partners were precipitated from lysates with streptavidin-agarose (SA). While gal-3 precipitated CD45 from control cells, there was a significant reduction in CD45 association with gal-3 in cells with reduced C2GnT-1.
All isoforms of CD45 can be decorated by complex N-glycans and core 2 O-glycans.16 Complex N-glycans and core 2 O-glycans both contain N-acetyl-lactosamine sequences, the preferred glycan ligands recognized by gal-3.10 Complex N-glycans have been identified as critical for gal-3 binding to some glycoprotein receptors on T cells.19 We analyzed SUDHL-6 and SUDHL-9 cells for complex N-glycan expression by flow cytometry with the plant lectin PHA-L and the 2 cell lines demonstrated comparable binding of PHA-L (supplemental Figure 2B). To determine whether complex N-glycans were required for gal-3 binding to SUDHL-6 cells, we treated the cells with the mannosidase I inhibitor DMNJ to block processing of complex N-glycans, and confirmed the effectiveness of DMNJ treatment by reduction in PHA-L binding (supplemental Figure 2C). However, DMNJ treatment did not reduce the amount of gal-3 on the cell surface (supplemental Figure 2C), nor did DMNJ treatment reduce the amount of CD45 that coprecipitated with gal-3 (data not shown).
As complex N-glycans were not essential for gal-3 binding to CD45 on these cells, we asked whether core 2 O-glycans were important for gal-3 binding to CD45. Core 2 O-glycans result from the addition of branching GlcNAc residues to asialo-core 1 O-glycans by the enzyme C2GnT-1; the core 2 branch can then be elongated into polylactosamine sequences, the preferred ligand of gal-3.10,16 SUDHL-6, but not SUDHL-9, cells had abundant asialo-core 1 O-glycans, as detected by flow cytometry with the plant lectin PNA (Figure 5C). In addition, SUDHL-6 cells expressed C2GnT-1, while SUDHL-9 cells did not (Figure 5D). We also determined that the heterogeneity of CD45 on SUDHL-6 cells as shown in Figure 5A was because of glycosylation. When SUDHL-6 cells were treated with DMNJ and C2GnT-1 siRNA individually or simultaneously, we observed decreased CD45 heterogeneity by immunoblot (supplemental Figure 2D), substantiating that CD45 heterogeneity on SUDHL-6 was because of glycosylation by both complex N-glycans and core 2 O-glycans.
To determine whether core 2 O-glycans were necessary for gal-3 binding to SUDHL-6 cells, we reduced expression of C2GnT-1 using siRNA. The efficiency of the siRNA treatment was confirmed by RT-PCR (Figure 5E). Exogenous biotinylated gal-3 was added to SUDHL-6 cells with reduced C2GnT-1 expression, the bound exogenous gal-3 was precipitated from cell lysates with streptavidin-agarose, and CD45 in the precipitates was detected by immunoblotting. As shown in Figure 5F, reduction of C2GnT-1 expression decreased the amount of gal-3 bound to CD45. Thus, core 2 O-glycans, not complex N-glycans, are required for gal-3 binding to CD45 on DLBCL cells.
Gal-3 binding to CD45 modulates phosphatase activity
CD45 is the most abundant tyrosine phosphatase in B cells. For T cells, there is abundant evidence that intracellular CD45 phosphatase activity is regulated by the degree of CD45 clustering on the cell surface; CD45 phosphatase activity is reduced when CD45 is clustered.33 Another galectin family member, galectin-1, binds to CD45 on T cells and reduces phosphatase activity by clustering CD45, which is involved in galectin-1–mediated apoptosis.20,34,35 To evaluate whether gal-3 binding to CD45 on DLBCL cells affects intracellular phosphatase activity, exogenous gal-3 was added to SUDHL-6 and SUDHL-9 cells and tyrosine phosphatase activity in cell extracts was measured. Exogenous gal-3 significantly reduced phosphatase activity in SUDHL-6 cells, whereas phosphatase activity in SUDHL-9 cells, which was inherently lower than that of SUDHL-6 cells, was unaffected by exogenous gal-3 (Figure 6A). The inhibitory effect of gal-3 on SUDHL-6 cells was abrogated by the competitive inhibitor lactose, but not by sucrose, confirming that the reduction in CD45 phosphatase activity resulted from gal-3 binding to glycans on CD45 (Figure 6B). Galectin-1 bound to CD45 on SUDHL-6 cells, but did not affect phosphatase activity (data not shown), demonstrating the specificity of the gal-3 effect on these cells.
Exogenous gal-3 binding to CD45 reduces tyrosine phosphatase activity. (A) SUDHL-6 and SUDHL-9 cells were treated with 5μM gal-3 and intracellular tyrosine phosphatase activity determined spectrophotometrically. Exogenous gal-3 significantly decreased phosphatase activity in SUDHL-6 cells, but had no effect on phosphatase activity in SUDHL-9 cells (***P < .0001). (B) Gal-3 reduction of CD45 phosphatase activity is glycan dependent. Gal-3 was preincubated with 100mM lactose or sucrose or with buffer control for 15 minutes at 37°C before treatment of SUDHL-6 cells. Although treatment with gal-3 alone or gal-3 plus sucrose significantly reduced phosphatase activity (***P < .0001), no reduction in phosphatase activity was seen in cells treated with gal-3 plus lactose to block gal-3 binding. (C) Multimerization of gal-3 is required to reduce phosphatase activity. Gal-3C is the C-terminal domain of gal-3 that lacks the N-terminal peptide required for multimerization. SUDHL-6 cells were treated with gal-3 or gal-3C and assayed for phosphatase activity. Although gal-3 significantly reduced phosphatase activity (***P < .0001), gal-3C did not affect phosphatase activity. Values are mean ± SD for triplicates from 1 representative of 3 independent replicate experiments.
Exogenous gal-3 binding to CD45 reduces tyrosine phosphatase activity. (A) SUDHL-6 and SUDHL-9 cells were treated with 5μM gal-3 and intracellular tyrosine phosphatase activity determined spectrophotometrically. Exogenous gal-3 significantly decreased phosphatase activity in SUDHL-6 cells, but had no effect on phosphatase activity in SUDHL-9 cells (***P < .0001). (B) Gal-3 reduction of CD45 phosphatase activity is glycan dependent. Gal-3 was preincubated with 100mM lactose or sucrose or with buffer control for 15 minutes at 37°C before treatment of SUDHL-6 cells. Although treatment with gal-3 alone or gal-3 plus sucrose significantly reduced phosphatase activity (***P < .0001), no reduction in phosphatase activity was seen in cells treated with gal-3 plus lactose to block gal-3 binding. (C) Multimerization of gal-3 is required to reduce phosphatase activity. Gal-3C is the C-terminal domain of gal-3 that lacks the N-terminal peptide required for multimerization. SUDHL-6 cells were treated with gal-3 or gal-3C and assayed for phosphatase activity. Although gal-3 significantly reduced phosphatase activity (***P < .0001), gal-3C did not affect phosphatase activity. Values are mean ± SD for triplicates from 1 representative of 3 independent replicate experiments.
Gal-3, like other galectins, can form multimers such as dimer or pentamers that enhance binding avidity.17,36,37 Galectin-mediated multimerization of glycoprotein ligands is required for most effects of cell-surface galectins. To determine whether the reduction in phosphatase activity after addition of gal-3 to SUDHL-6 cells required gal-3 multimerization, we used gal-3C, a construct containing only the C-terminal CRD and lacking the N-terminal domain required for multimerization. We treated SUDHL-6 cells with gal-3C and measured phosphatase activity. Unlike intact gal-3 that reduced phosphatase activity, binding of gal-3C did not reduce phosphatase activity (Figure 6C). This indicates that gal-3 binding to CD45 and reduction of phosphatase activity involves clustering of CD45 by multimeric gal-3.
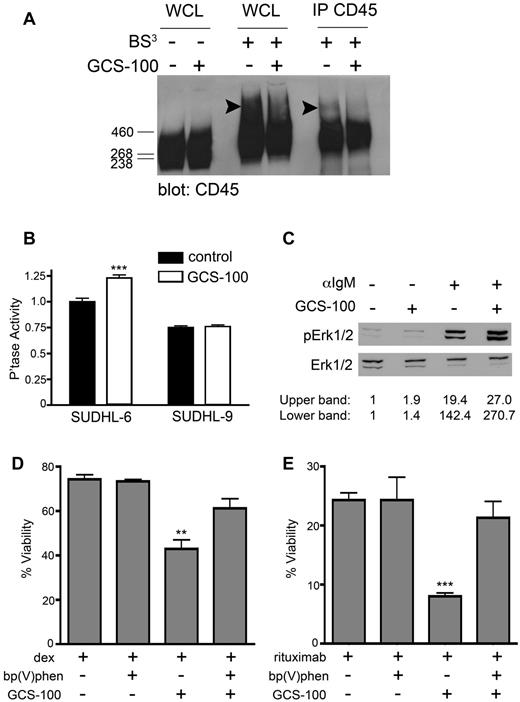
We directly assessed whether gal-3 binding to CD45 facilitates clustering. Cell-surface proteins on SUDHL-6 cells treated with or without GCS-100 were cross-linked with the nonreducible cross-linking agent BS.3 As shown in Figure 7A, BS3 cross-linked CD45 from whole-cell lysates of control cells migrated as a high-molecular-weight smear, while BS3 cross-linked CD45 from lysates of cells pretreated with GCS-100 lacked the highest molecular-weight bands seen in the control cells. Similarly, immunoprecipitated cross-linked CD45 from control SUDHL-6 cells contained high-molecular-weight bands that were not detectable in cross-linked CD45 immunoprecipitated from GCS-100–treated cells.
Removal of endogenous gal-3 from CD45 increases phosphatase activity to potentiate DLBCL cell death. (A) Removal of cell-surface gal-3 alters CD45 clustering. SUDHL-6 cells were treated with 75 μg/mL GCS-100 or buffer control for 1 hour and cell-surface glycoproteins were immediately cross-linked with the nonreducible cross-linker BS3. CD45 was immunoprecipitated from cell lysates and resolved by 3%-8% Tris-acetate SDS-PAGE. In control-treated cells, high-molecular-weight clusters of CD45 are present (arrowheads), which are diminished when cells are treated with GCS-100. (B) Removal of cell-surface gal-3 increases phosphatase activity. SUDHL-6 and SUDHL-9 cells were treated with 75 μg/mL GCS-100 and phosphatase activity measured (***P < .0001, values are mean ± SD of triplicates from 1 representative of 6 independent replicate experiments). (C) Removal of cell-surface gal-3 alters downstream signaling regulated by via CD45 phosphatase. SUDHL-6 cells were treated with 75 μg/mL GCS-100 or buffer control for 30 minutes before treatment with 10 μg/mL anti-IgM for 1 minute. Lysates were immunoblotted with anti-phospho-Erk and anti-Erk. The levels of Erk and phospho-Erk were determined by densitometry and the levels of phospho-Erk were normalized to Erk expression. Increased Erk phosphorylation after anti-IgM cross-linking was observed in GCS-100–treated cells. (D-E) GCS-100 sensitization to cell death requires phosphatase activity. SUDHL-6 cells were treated with the phosphatase inhibitor 12.5nM bp(V)phen for 1 hour, followed by 75 μg/mL GCS-100 for 1 hour, and (D) dex or (E) rituximab for 24 hours. Viability was determined as described in “Methods.” **P < .001, ***P < .0001, values are mean ± SD of triplicates from 1 representative of 3 independent replicate experiments for each treatment.
Removal of endogenous gal-3 from CD45 increases phosphatase activity to potentiate DLBCL cell death. (A) Removal of cell-surface gal-3 alters CD45 clustering. SUDHL-6 cells were treated with 75 μg/mL GCS-100 or buffer control for 1 hour and cell-surface glycoproteins were immediately cross-linked with the nonreducible cross-linker BS3. CD45 was immunoprecipitated from cell lysates and resolved by 3%-8% Tris-acetate SDS-PAGE. In control-treated cells, high-molecular-weight clusters of CD45 are present (arrowheads), which are diminished when cells are treated with GCS-100. (B) Removal of cell-surface gal-3 increases phosphatase activity. SUDHL-6 and SUDHL-9 cells were treated with 75 μg/mL GCS-100 and phosphatase activity measured (***P < .0001, values are mean ± SD of triplicates from 1 representative of 6 independent replicate experiments). (C) Removal of cell-surface gal-3 alters downstream signaling regulated by via CD45 phosphatase. SUDHL-6 cells were treated with 75 μg/mL GCS-100 or buffer control for 30 minutes before treatment with 10 μg/mL anti-IgM for 1 minute. Lysates were immunoblotted with anti-phospho-Erk and anti-Erk. The levels of Erk and phospho-Erk were determined by densitometry and the levels of phospho-Erk were normalized to Erk expression. Increased Erk phosphorylation after anti-IgM cross-linking was observed in GCS-100–treated cells. (D-E) GCS-100 sensitization to cell death requires phosphatase activity. SUDHL-6 cells were treated with the phosphatase inhibitor 12.5nM bp(V)phen for 1 hour, followed by 75 μg/mL GCS-100 for 1 hour, and (D) dex or (E) rituximab for 24 hours. Viability was determined as described in “Methods.” **P < .001, ***P < .0001, values are mean ± SD of triplicates from 1 representative of 3 independent replicate experiments for each treatment.
If removing cell-surface gal-3 from CD45 reduced CD45 clustering, and CD45 clustering is known to decrease CD45 phosphatase activity,33 we asked whether removal of cell-surface gal-3 would increase CD45 phosphatase activity. Treatment of SUDHL-6 cells with GCS-100 resulted in a significant increase in tyrosine phosphatase activity, while treatment of SUDHL-9 cells with GCS-100 did not affect phosphatase activity (Figure 7B). These results demonstrate that endogenous cell-surface gal-3 binding to CD45 promotes CD45 multimerization and reduces tyrosine phosphatase activity.
We confirmed that the GCS-100–mediated increase in tyrosine phosphatase activity affected signaling pathways downstream of CD45. We examined the B-cell receptor (BCR) signaling, a pathway that involves CD45 phosphatase activity.22,38 SUDHL-6 cells were treated with GCS-100, BCR signaling was stimulated by cross-linking with anti-IgM, and Erk activation was analyzed by immunoblot. Removal of cell-surface gal-3 from DLBCL cells before BCR cross-linking with anti-IgM resulted in more robust activation of Erk, as determined by increased Erk phosphorylation, compared with control cells treated with anti-IgM alone (Figure 7C). We also examined Lyn, a substrate of CD45 phosphatase in B cells22 ; GCS-100 treatment of SUDHL-6 cells resulted in decreased phosphorylation of Lyn at inhibitory residue Y507, compared with Lyn from control-treated cells, also consistent with increased CD45 phosphatase activity (supplemental Figure 4).
To determine whether the increase in CD45 phosphatase activity resulting from removal of cell-surface gal-3 was important for GCS-100–mediated sensitization of gal-3+ DLBCL cells to apoptosis-inducing agents (Figure 3), SUDHL-6 cells were treated with the tyrosine phosphatase inhibitor bp(V)phen20 before treatment with GCS-100 and dex or rituximab. Addition of bp(V)phen alone decreased tyrosine phosphatase activity in SUDHL-6 cells without decreasing cell viability (data not shown). While GCS-100 treatment sensitized the cells to dex and rituximab, as seen in Figure 3, addition of bp(V)phen abrogated this effect of GCS-100, and restored resistance to both dex and rituximab (Figure 7D-E). Thus, GCS-100–mediated removal of cell-surface gal-3 from CD45-enhanced CD45 phosphatase activity, and this enhanced phosphatase activity was essential for GCS-100–mediated sensitization of the cells to cell death. Taken together, these data identify novel roles for cell-surface gal-3 and CD45 in promoting apoptosis resistance of DLBCL cells.
Discussion
The resistance of lymphoma cells to apoptosis induced by chemotherapeutic agents is a major obstacle in the treatment of DLBCL.3,4 We propose that expression and cell-surface localization of gal-3, and interaction of cell-surface gal-3 with CD45 to regulate CD45 phosphatase activity, is a novel mechanism of apoptosis resistance in DLBCL. In the present study, when the interaction of gal-3 and CD45 on DLBCL cells was disrupted by removing cell-surface gal-3 with GCS-100, cells were sensitized to apoptosis induced by a variety of agents (Figure 3). Furthermore, signaling from CD45 phosphatase, which was activated by removing gal-3 from CD45 (Figure 7B), was required for this sensitization to apoptosis (Figure 7D and E). These findings indicate that cell-surface gal-3 may act as an upstream “apoptotic block” in DLBCL, the majority of which express gal-3,4,6-9 and that disrupting the gal-3/CD45 interaction may enhance sensitivity of these cells to a range of chemotherapeutic agents. Moreover, this work identifies a novel role for CD45 in DLBCL survival.
Importantly, this study directly examined the role of cell-surface gal-3 in regulating DLBCL cell survival. Removal of only cell-surface gal-3 by GCS-100 was sufficient to sensitize cells to apoptosis (Figure 3), indicating that the subpopulation of gal-3 at the cell surface could exert an antiapoptotic function. Previous studies examining the antiapoptotic activity of gal-3 have concentrated on cytoplasmic gal-3 interacting with Bcl-2 at mitochondria, while cell-surface gal-3 has been implicated in other functions, such as regulating TCR signaling and promoting T-cell death or promoting tumor cell adhesion and metastasis.13,19,23,39,40 However, a recent study found that gal-3 acts at the cell surface to promote survival of colon adenocarcinoma cells.41 While cytoplasmic gal-3 may also have a role in promoting survival of DLBCL cells, our findings demonstrate a clear role for cell-surface gal-3 in DLBCL cell resistance to apoptosis, and cell-surface gal-3 may be an accessible and thus more tractable therapeutic target.
We propose that gal-3 binding to CD45 generates a cell-surface galectin-glycoprotein lattice with subsequent reduction in phosphatase activity and downstream regulation of signaling pathways involved in apoptosis (Figure 7). This is the first report of a role for CD45 in the survival of DLBCL cells, and implies that CD45 phosphatase activity may also be a therapeutic target in DLBCL. Gal-3 binding to CD45 creates high-molecular-weight clusters of CD45 that are dispersed by removal of gal-3 with GCS-100 (Figure 7A), and gal-3 binding to CD45 regulates phosphatase activity (Figures 6A, 7B). This is consistent with prior work showing that CD45 multimerization, either by galectin-1 binding or by modulating CD45 glycosylation, down-regulates phosphatase activity,20,33,42 although this is the first report of gal-3 having this effect. Remarkably, gal-3− SUDHL-9 cells had inherently lower phosphatase activity compared with gal-3+ SUDHL-6 cells (Figure 6A). Furthermore, phosphatase activity of SUDHL-6 cells could be enhanced, leading to increased susceptibility to apoptosis, by disrupting the gal-3/CD45 interaction with GCS-100, while phosphatase activity of SUDHL-9 cells could not be increased in this manner. Together, this suggests that SUDHL-6 cells have “activatable” CD45, while SUDHL-9 cells have “non-activatable” CD45.
CD45 glycosylation is critical in regulating the interaction with gal-3. Notably, inhibiting formation of core 2 O-glycans reduced gal-3 binding to CD45 on DLBCL cells, while inhibiting formation of complex N-glycans did not reduce gal-3 binding (Figure 5, supplemental Figure 2). These data indicate that gal-3 binds to core 2 O-glycans on DLBCL cells to cluster CD45 and regulate phosphatase activity. While previous studies have identified N-glycans as critical for gal-3 binding to glycoproteins, including CD45, on T cells,19,24 core 2 O-glycans may be the primary ligands for gal-3 on DLBCL cells.
In analyzing DLBCL cell lines, we found that gal-3 was expressed by cells that also expressed a heterogeneous, higher molecular-weight population of CD45, indicating extensive glycosylation and glycan heterogeneity; SUDHL-6 expressed both gal-3 and high-molecular-weight CD45, while SUDHL-9 did not express gal-3 and had less CD45 heterogeneity (Figure 5). Importantly, we also observed a similar trend regarding CD45 heterogeneity, gal-3 expression, and phosphatase activity in primary DLBCL tissue, albeit with a small sample size (supplemental Figure 5). Does CD45 glycosylation influence gal-3 expression or vice versa? Recent studies provide compelling examples where the expression or loss of a single lectin results in altered glycosylation and vice versa,43-45 suggesting that this could be the case with gal-3 and CD45 glycosylation in DLBCL cells.
Although antiapoptotic mechanisms have been identified for subpopulations of DLBCL,46-48 there is no consistent mechanism of apoptosis resistance in DLBCL. However, gal-3 is consistently reported to be expressed by the majority of primary DLBCL,4,6-9 regardless of genetic subtype. As removal of cell-surface gal-3 with GCS-100 sensitized cells to a variety of agents with distinct apoptotic mechanisms (Figure 3), cell-surface gal-3 may act as a general or upstream apoptotic block in DLBCL. Because GCS-100 removes cell-surface gal-3 (Figure 2),18 this indicates that GCS-100 could be a useful adjunct in therapies for DLBCL. Unlike other gal-3 inhibitors that have been proposed as cancer therapeutics,11,49 for which the mechanism(s) and site(s) of action have not been thoroughly studied, it is clear from the present work that GCS-100 acts at the cell surface to remove gal-3 from CD45, regulating phosphatase activity to permit apoptotic signaling. In addition to the current study demonstrating that GCS-100 sensitized DLBCL cells to apoptosis, prior work has shown that GCS-100 promotes apoptosis of other types of hematopoietic tumor cells, such as multiple myeloma, directly or in combination with chemotherapeutic agents.12,13 In addition, GCS-100 activated tumor infiltrating lymphocytes and promoted tumor rejection in a mouse model.18 GCS-100 has already been in phase 1 clinical trials, and demonstrated an acceptable human safety profile, prompting initiation of subsequent trials in several types of hematopoietic malignancies.13,50-52 Thus, GCS-100 may warrant reexamination as a promising adjunct therapy in DLBCL. Collectively, the data presented here identify the interaction of cell-surface gal-3 with CD45, regulating CD45 phosphatase activity, as a novel mechanism of apoptosis resistance in DLBCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sandra Thiemann for helpful discussions and Kenneth Dorshkind, Michael Teitell, and C. Fred Brewer for critical reading of the manuscript.
This work was supported by grants from the Ron and Maddie Katz Family Foundation, UCLA's Jonsson Comprehensive Cancer Center, the National Institutes of Health (NIH) R21 CA139368, NIH Career Development Award 5P50 CA96888 P.I. R. Ambinder (L.G.B.), the Terry Fox Foundation 019001 (R.D.G.), and NIH T32 CA009056 (M.C.C.).
National Institutes of Health
Authorship
Contribution: M.C.C. designed the research, performed experiments, analyzed and interpreted results, and contributed to manuscript preparation; M.P. designed research and performed experiments; D.K.H. and F.-T.L. contributed to experimental design and provided reagents; S.d.V., R.D.G., and J.S. identified clinical samples and assisted with data collection and image analysis; and L.G.B. directed the study, participated in the experimental design and data analysis, and contributed to manuscript preparation.
Conflict-of-interest disclosure: L.G.B. was on the Scientific Advisory Board for Prospect Therapeutics (2007-2008). The remaining authors declare no competing financial interests.
Correspondence: Dr Linda G. Baum, Department of Pathology and Laboratory Medicine, UCLA School of Medicine, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: lbaum@mednet.ucla.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal