Abstract
HIV-1 infections are generally initiated at mucosal sites. Thus, IgA antibody, which plays pivotal roles in mucosal immunity, might efficiently prevent HIV infection. However, mounting a highly effective HIV-specific mucosal IgA response by conventional immunization has been challenging and the potency of HIV-specific IgA against infection needs to be addressed in vivo. Here we show that the polymeric IgA form of anti-HIV antibody inhibits HIV mucosal transmission more effectively than the monomeric IgA or IgG1 form in a comparable range of concentrations in humanized mice. To deliver anti-HIV IgA in a continual manner, we devised a hematopoietic stem/progenitor cell (HSPC)–based genetic approach using an IgA gene. We transplanted human HSPCs transduced with a lentiviral construct encoding a class-switched anti-HIV IgA (b12-IgA) into the humanized bone marrow-liver-thymus (BLT) mice. The transgene was expressed specifically in B cells and plasma cells in lymphoid organs and mucosal sites. After vaginal HIV-1 challenge, mucosal CD4+ T cells in the b12-IgA–producing mice were protected from virus-mediated depletion. Similar results were also obtained in a second humanized model, “human immune system mice.” Our study demonstrates the potential of anti-HIV IgA in immunoprophylaxis in vivo, emphasizing the importance of the mucosal IgA response in defense against HIV/AIDS.
Introduction
Immunoglobulin A (IgA), the most abundant isotype secreted at mucosal sites, plays critical roles in mucosal immune responses by blocking viral attachment and crossing epithelial barriers to neutralize virus infectivity.1 Hence, it could be effective to provide IgA as a protection against HIV infection. The inhibitory effect of IgA on transepithelial entry of HIV has been studied using polarized epithelial cell line in vitro.2,3 However, despite its potential importance, the potency of HIV-specific IgA has yet to be precisely addressed in animal models.
Because of the various immune evasion mechanisms of HIV,4 no vaccine yet induces a highly effective anti-HIV antibody response, not to mention IgA. Moreover, it has been found that HIV-1 inhibits IgA class-switching in B cells through long intercellular conduits emitted from virus-infected macrophages.5 Thus the elicitation of a highly effective anti-HIV IgA response will probably be challenging using conventional immunization.
Several potent broadly neutralizing antibodies (bNAbs) to HIV-1 have been recovered from infected subjects as monoclonal antibodies (mAbs),6-8 and b12 (IgG1) is one of these.9 Using these potent mAbs, genetic approaches have been explored as an alternative anti-HIV prophylaxis. Viral vector-mediated transfer of genes encoding neutralizing antibody (NAb) or antibody-like immunoadhesins have shown efficacy in preclinical models.10-12 Besides anti-HIV antibody, targeted gene knockdown or RNA-based anti-HIV therapies have also been attempted in humanized mice and tested in several clinical trials.13-18 Suppressive effect of these approaches on HIV infection support the potential of genetic engineering to control the HIV/AIDS epidemic. Recently, HSPC-mediated antibody gene transfer for HIV has been explored by Joseph et al in a humanized mouse model and they demonstrated immunoprophylaxis by the IgG NAb 2G12, the expression of which was directed by a constitutive promoter.19 Because of their unlimited regenerative ability and their capacity for multilineage differentiation, HSPCs are an attractive vehicle for a gene therapy. However, for the same reason, it would be highly desirable to have selective transgene expression restricted in specific cell lineages or developmental stages.
Here we elucidate the role of anti-HIV IgA in vivo and demonstrate that anti-HIV IgA isotype is more potent than its IgG1 counterpart in inhibiting infection after mucosal HIV challenge in humanized mice. We also found that in vivo it is polymeric IgA (pIgA) that dominated this protective effect rather than monomeric IgA (mIgA). Furthermore, we attempted to provide anti-HIV IgA to humanized mice through HSPC-mediated gene transfer in a cell/tissue-specific and development-stage-specific manner. The b12-IgA–transduced humanized mice were protected from HIV-induced mucosal CD4+ T-cell depletion after mucosal challenge with HIV even at low concentrations of b12-IgA in plasma and mucosal sites (< 20 ng/mL). The results show that implantation of an anti-HIV IgA bNAb gene into HSPCs can provide anti-HIV mucosal immunity by actively reprogramming the immune system, demonstrating the potential for IgA and mucosal immunity in HIV/AIDS immunoprophylaxis.
Methods
Construction of lentivirus vector encoding human IgA2 b12
The heavy chain of IgA2 b12 was constructed by combining the variable domain of b12-IgG1 heavy chain with the constant domains of human IgA2 (VHCalpha2m[1]). The expression cassette of the IgA2 b12 included the chimeric heavy chain IgA2 b12, the κ light chain of b12 and the human IgJ chain linked by 2A sequences. The expression cassette was inserted into various lentivirus vectors including FUW,20 pHAGE621 with the human IgL chain promoter (EEK).22 For the control vectors, eGFP (FUGW)20 or ZsGreen (pHAGE6-EEK-Luc-ZsGr) were used.
In vitro neutralization assay
We performed a pseudovirus neutralization assay using TZM-bl cells and pseudotyped viruses generated by cotransfection of HEK239T cells with an Env expression plasmid (SF162.LS, accession no. EU123924), and a replication-defective backbone plasmid (pSG3delta env, accession no. L02317) as previously described.23
Passive transfer of purified antibodies to humanized mice before HIV challenge
For passive antibody transfer experiments, NOD.Cg-PrkdscidIL2rgtm1Wjl/SzJ (NOD/SCIDIL2rγ−/−, NSG) mice were transplanted intraperitoneally with 3 × 106 human PBMCs (AllCells) that were activated by PHA (5 μg/mL) for 4 days before transplantation (NSG-hu mice). Human cell engraftments in the mice were assessed at 3 to 5 weeks after transplantation. NSG mice were transplanted intraperitoneally with 3 × 106 human PBMCs (AllCells) that were activated by PHA (5 μg/mL) for 4 days before transplantation (NSG-hu mice). Human cell engraftments in the mice were assessed at 3 to 5 weeks after transplantation. NSG-hu mice were injected intravenously with purified antibodies through tail vein 4 hours before HIV challenge. Human IgG/κ (Bethyl laboratory.) were used as control antibody (200 μg per mouse). Each mouse was administered with 200 μg of antibody diluted in 100 μL of sterile PBS; b12-IgA2 with monomer and polymer (100 μg of pIgA and 100 μg of mIgA); b12-IgG1 (20 μg of b12-IgG1 and 180 μg of control IgG/kappa); b12-IgA2 monomer only (200 μg of mIgA).
HIV-1 virus production and mucosal challenge
HIV-1 (JR-CSF) was obtained through the NIAID consortium and other authorized sources. Human 293T cells were used to allow virus particle formation and accumulation in the supernatant. For mucosal HIV challenge, female mice were infected through the vaginal mucosal route (IVAG). The mice were treated with 2 mg of progesterone (Depo-Provera; Pharmacia & Upjohn Diagnostics) per mouse subcutaneously in a 100 μL volume at 3 to 7 days before infection. On the day of infection, the mice were anesthetized and infected IVAG with the virus (170 ng of p24, or a 50% tissue culture infective dose [TCID50] of 170 000) in maximum volume of 20 μL using a micropipette tip by surface application without apparent trauma while being maintained anesthesia. Uninfected animals received PBS intravaginally. For the assessment of plasma viral load, plasma collected from humanized mice was diluted to 1 mL with human serum and each sample was examined using Abbott real-time viral load test.
Generation of humanized BLT mice
NSG mice were purchased from The Jackson Laboratory and maintained in the animal facilities at California Institute of Technology and University of California, Los Angeles (UCLA), in accordance with National Institutes of Health guidelines. Humanized bone marrow/liver/thymus (hu-BLT) mice were generated as previously described.24,25 Briefly, 6- to 8-week-old mice were implanted with a fragment of human fetal thymus and Matrigel-solidified CD34+ (0.5 × 106) and CD34− cells (4.5 × 106) that were isolated from human fetal liver under the kidney capsule. To generate transgene-transduced hu-BLT mice, the CD34+ cells were infected with lentiviral vector in the presence of retronectin at multiplicity of infection (MOI) of 0.2 to 3 for overnight before the transplantation. Three weeks after implantation, the mice were sublethally irradiated (325cGy by 60Co irradiation) and were transplanted with 1 × 106 lentivector-transduced autologous human fetal CD34+ cells intravenously. Human cell engraftment was assessed periodically by monitoring the percentage of human CD45+ cells in the peripheral blood samples.
Lymphocyte isolation from intestine, genital tract, and lung
The entire mouse gastrointestinal (GI) tract or genital tract were isolated and dissected into pieces smaller than 0.5 cm and washed with 45 mL ice-cold PBS at least 4 times until the residual fecal material and mucus were completely removed. For the intraepithelial lymphocytes (IEL) isolation from the gut and genital tract, the tissues were then incubated in 25 mL and 12.5 mL of freshly prepared cell dissociation solution (Ca++ and Mg++ free HBSS, 5mM EDTA, 10mM HEPES, respectively) for 20 minutes at 37°C with a slow rotation at 100 rpm. The supernatants were recovered and kept on ice. The tissues were saved for the lamina propria lymphocytes (LPL) isolation. For the LPL isolation from the gut and genital tract, the tissues were further incubated in 25 mL and 12.5 mL of LPL isolation solution (for gut, RPMI with 10% fetal bovine serum, 0.5 mg/mL collagenase type II; for genital tract, RPMI with 10% fetal bovine serum, 0.5 mg/mL collagenase type II, 0.1 mg/mL DNase type I, 25mM HEPES, 5mM β-mercaptoethanol, respectively) for 30 minutes at 37°C with a slow rotation at 100 rpm. The mixture was then vortexed for 30 seconds and the supernatant was collected using a 40-μm cell strainer. If necessary, the incubation was repeated twice with fresh LPL isolation solution. The IELs and LPLs were isolated from the supernatants through a 2-step (40/80%) percoll gradient centrifugation. Lung lymphocytes were recovered from the bronchoalveolar lavage fluid by centrifugation at 11g in a microcentrifuge.
Results
The IgA form of anti-HIV antibody is more effective than its IgG1 counterpart in inhibiting HIV-1 infection
To investigate the potency of anti-HIV IgA isotype antibody for inhibiting HIV infection, the protective activity of a human IgA2 form of b12 (b12-IgA2) antibody was examined in comparison with a matched IgG1 form (b12-IgG1). Human IgA2 subclass was used in this study because IgA2 is more resistant than IgA1 to digestion by bacteria abundant in mucosal compartments.1 We constructed a class-switched b12-IgA2 by combining the variable domains of the heavy and light chains from b12-IgG1 and constant domains of IgA2 heavy chain. To verify that our b12-IgA construct is functionally intact, the recombinant monoclonal antibodies were purified (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and the neutralizing activity of monomeric b12-IgA2 and b12-IgG1 was assessed in vitro using an HIV pseudotype as target. The recombinant monomeric b12-IgA2 and b12-IgG1 were equally efficient at neutralization in vitro (Figure 1A) in accordance with a previous report.26
HIV-1 infection in humanized mice passively infused with b12-IgA or b12-IgG isotype. (A) Neutralization activity of anti-HIV human mAb b12-IgA. Reporter cell line TZM-bl cells that express CD4, CXCR4, CCR5, and a Tat-responsive reporter gene for luciferase were infected with 200 TCID50 of replication-defective pseudovirus containing Env (SF162.LS) in the presence of various concentrations of anti-HIV monoclonal antibodies. Neutralization activity was measured by the reduction in luciferase reporter gene expression after a single round of pseudovirus infection in TZM-bl cells in triplicate. b12-IgG1 and the recombinant b12-IgA2 were compared at indicated concentrations. (B) Antibody level in circulation after passive transfer. NSG-hu mice were injected intravenously with various concentrations of purified b12-IgA2 (pIgA: mIgA = 1:1, mass ratio) or b12-IgG1 and the blood was collected after 4 hours. The plasma antibody concentrations were measured using ELISA. (n = 4-6, mean ± SEM). (C-D) Concentrations of b12 antibodies in plasma (C) or genital secretions (D) at the time of challenge. NSG-hu mice were injected intravenously with either 200 μg of b12-IgA2 or 20 μg of b12-IgG1 per mouse. Blood and genital secretions were collected after 4 hours. (E-I) Peripheral CD4+ T cell loss after HIV-1 challenge in NSG-hu mice injected with different b12 antibody isotypes; (E) purified human IgG/κ control antibody (hIgG/κ); (F) b12-IgA2 includes both monomeric and polymeric IgA as described in (B), (b12IgA2 [M+P]); (G) b12-IgG1; (H) b12-IgA2 monomer only (b12IgA2 [M]). Mice were challenged intravaginally with HIV-1JR-CSF. (I) Average percent CD4+ T cells in CD3 T cells in PBMCs at each time points (n = 5-9). Statistical analysis was performed using unpaired t test. (*P = .0313 and ***P < .0001 between IgA [M+P] +HIV and control Ab [hIgG/κ] + HIV). (J) Plasma viral loads in HIV infected NSG-hu mice. HIV viral RNA was measured by Abbott HIV viral load test. Dotted line indicates the detection limit. (N/D indicates not detected; n = 4, indicated P values are from unpaired t test between Ab-treated groups with HIV and hIgG/κ-treated groups with HIV.)
HIV-1 infection in humanized mice passively infused with b12-IgA or b12-IgG isotype. (A) Neutralization activity of anti-HIV human mAb b12-IgA. Reporter cell line TZM-bl cells that express CD4, CXCR4, CCR5, and a Tat-responsive reporter gene for luciferase were infected with 200 TCID50 of replication-defective pseudovirus containing Env (SF162.LS) in the presence of various concentrations of anti-HIV monoclonal antibodies. Neutralization activity was measured by the reduction in luciferase reporter gene expression after a single round of pseudovirus infection in TZM-bl cells in triplicate. b12-IgG1 and the recombinant b12-IgA2 were compared at indicated concentrations. (B) Antibody level in circulation after passive transfer. NSG-hu mice were injected intravenously with various concentrations of purified b12-IgA2 (pIgA: mIgA = 1:1, mass ratio) or b12-IgG1 and the blood was collected after 4 hours. The plasma antibody concentrations were measured using ELISA. (n = 4-6, mean ± SEM). (C-D) Concentrations of b12 antibodies in plasma (C) or genital secretions (D) at the time of challenge. NSG-hu mice were injected intravenously with either 200 μg of b12-IgA2 or 20 μg of b12-IgG1 per mouse. Blood and genital secretions were collected after 4 hours. (E-I) Peripheral CD4+ T cell loss after HIV-1 challenge in NSG-hu mice injected with different b12 antibody isotypes; (E) purified human IgG/κ control antibody (hIgG/κ); (F) b12-IgA2 includes both monomeric and polymeric IgA as described in (B), (b12IgA2 [M+P]); (G) b12-IgG1; (H) b12-IgA2 monomer only (b12IgA2 [M]). Mice were challenged intravaginally with HIV-1JR-CSF. (I) Average percent CD4+ T cells in CD3 T cells in PBMCs at each time points (n = 5-9). Statistical analysis was performed using unpaired t test. (*P = .0313 and ***P < .0001 between IgA [M+P] +HIV and control Ab [hIgG/κ] + HIV). (J) Plasma viral loads in HIV infected NSG-hu mice. HIV viral RNA was measured by Abbott HIV viral load test. Dotted line indicates the detection limit. (N/D indicates not detected; n = 4, indicated P values are from unpaired t test between Ab-treated groups with HIV and hIgG/κ-treated groups with HIV.)
To compare the protective activity of anti-HIV IgA and IgG in vivo in a range of comparable concentrations, we passively transferred b12-IgA2 or b12-IgG1 antibody to humanized mice before virus challenge. Because of a difference between the half-life of human IgA2 and that of human IgG1 in plasma, the dose of the monoclonal antibodies administered was adjusted so that the plasma concentrations of the 2 isotypes of antibody were comparable at the time of virus challenge. Various amounts of purified monoclonal antibodies were intravenously injected into humanized NOD/SCIDγC−/−(NSG) mice that were previously transplanted with human PBMCs (NSG-hu mouse model). We used a mixture of purified monomeric and polymeric b12-IgA (polymer: monomer = 1:1 mass ratio, 0.48:1 molar ratio) because normal human serum IgA includes both polymeric and monomeric IgA.
We assessed the concentrations of these antibodies in the circulation of the mice at 4 hours after transfer (Figure 1B). Purified human b12-IgA2 was more rapidly cleared from mouse plasma than its IgG1 counterpart, correlating with the differences between the half-lives of the isotypes in humans (average half-life, 4.5 days for IgA2 and 36.3 days for IgG1).27,28 The humanized NSG mice injected with 20 μg of b12-IgG1 per mouse yielded comparable plasma concentrations of antibody to mice injected with 200 μg b12-IgA2 after 4 hours (Figure 1B-C). At this dose, transferred b12-IgA2 and b12-IgG1 exhibited comparable concentrations of antibody also in genital secretions (Figure 1D).
The difference in blood antibody concentrations between the 2 isotypes, presumably because of the difference in half-life of the 2 antibodies, was also observed in mice harboring genetically modified cells transduced with the 2 antibody genes. The plasma concentration of b12-IgA antibody produced from b12-IgA–transduced mice was much lower than the plasma concentration of b12-IgG in b12-IgG–transduced mice (supplemental Figure 2).
The protective effect of b12-IgA and b12-IgG was compared after HIV-1 challenge. Human serum and genital secretions contain both mIgA and pIgA, although pIgA is predominant in intestinal secretions.29 We injected a mixture of purified polymeric and monomeric b12-IgA2 (1:1 = polymer: monomer, mass ratio, M+P), purified monomeric b12-IgA2 only (M), purified b12-IgG1 or control antibody into separate groups of humanized mice. Because mucosal transmission through the genital tract or rectum is the principal route of HIV-1 infection, we challenged the animals with CCR5-tropic HIV-1 (HIV-1JR-CSF) mucosally by the intravaginal route. HIV infection was assessed by loss of peripheral CD4+ T cells. After HIV challenge, peripheral CD4+ T cells were depleted in the control antibody-injected mice (Figure 1E,I). However, 6 of 9 mice administered b12-IgA2 antibodies that included both monomeric and polymeric IgA maintained CD4+ T cells after virus challenge (Figure 1F,I), whereas most of the mice injected with b12-IgG1 (6 of 7) showed a reduction of CD4+ T cells down to less than 50% within 14 days (Figure 1G,I). The group with multimeric (monomeric+polymeric) IgA had significantly higher peripheral CD4+ T cells than the control Ab-treated group (P = .0313 at day 21, P < .0001 at day 28). Statistical analysis also showed that the group of mice administered multimeric IgA were significantly more probable to maintain CD4+ T cells than the IgG treated group within 3 weeks of virus challenge (χ2 = 5.130, degrees of freedom (df) = 1, P = .0235). Monomeric b12-IgA2 only could not prevent viral infection as effectively as did the mixture of monomer and polymer (Figure 1H,I), indicating the importance of pIgA in mucosal protection. However, we cannot entirely exclude the possibility of a synergistic effect of pIgA and mIgA because we could not include a group treated with pIgA only because of the limited amount of pIgA we could purify.
Consistent with the CD4+ T cell loss, the viral loads in plasma were below the detection limit in all mice examined in the group treated with the multimeric b12-IgA2 (Figure 1J). Plasma viremia was reduced in mouse groups treated with b12-IgG1 or b12-IgA2 monomer only but the reduction was not statistically significant compared with the control Ab-treated group. These results demonstrate that in a comparable range of concentrations, the multimeric IgA of anti-HIV antibody prevents mucosal virus infection more efficiently than its IgG counterpart in vivo and that pIgA predominantly mediates the protective effects.
b12-IgA gene transfer to HSPC and its B-cell development
We further investigated the potential of anti-HIV IgA antibody against HIV mucosal transmission in humanized mice expressing anti-HIV IgA endogenously. To this end, we used an approach in which the transplants of genetically engineered HSPCs with b12-IgA gene develop into B-lineage cells producing the antibody in vivo.
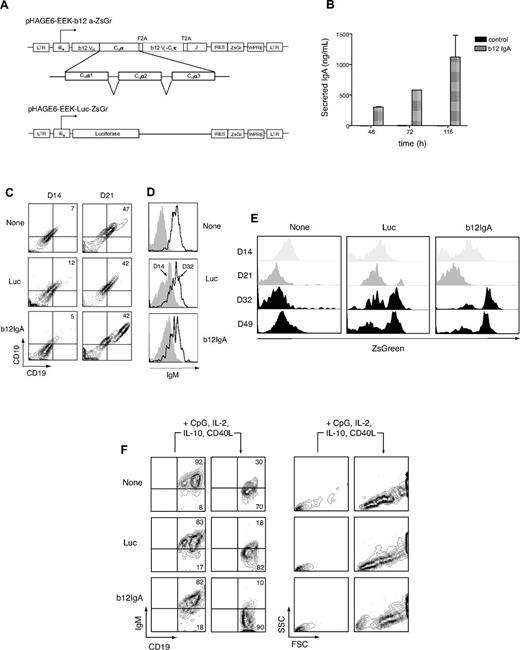
First, we constructed a lentiviral vector directing synthesis of the secretory heavy and light chain of class-switched b12-IgA2 and the immunoglobulin J chain (Figure 2A). To direct B-cell lineage-specific transgene expression, we used the human immunoglobulin light (IgL) chain κ promoter/enhancer, EEK, which was previously described to induce transgene expression in B cells in vitro.22 ZsGreen was provided as a reporter gene for either the b12-IgA or a luciferase transgene (negative control; Figure 2A). After transfection with the construct, 293T cells produced b12-IgA antibody in the culture supernatant verifying the expression and secretion of the antibody in human cells (Figure 2B). Secondly, to test whether the HSPCs transduced with a lentiviral vector carrying b12-IgA undergo normal differentiation to mature B-cells, we examined B-cell development using a 2-stage B lymphopoiesis culture system previously developed in this laboratory.22 Human cord blood CD34+ HSPCs were first transduced with the lentivector, then primed in the presence of cytokines for B-cell lineage commitment and finally cocultured with murine stromal cells, MS5, for several weeks. Transduced HSPCs developed into CD10+CD19+ pro-B cells (Figure 2C) and then further developed to surface IgM+ B cells with light chain genes rearranged (Figure 2D). Under control of the B cell–specific IgL promoter, transgene expression in CD19+ B lineage cells was evident after 5 weeks of coculture, when cells started expressing IgM. Transgene expression was highly B cell–specific as well as being developmental stage-specific (Figure 2E). In response to activation signals, some of the B-cell population developed into cells with reduced IgM expression and increased forward and side-scatter in flow cytometry, phenotypic characteristics of plasmablasts, suggesting that lentiviral expression of the b12-IgA transgene did not alter normal B lymphopoiesis (Figure 2F).
Development of human B cells from HSPCs transduced with b12-IgA gene in vitro. (A) Schematic representation of the lentiviral constructs. Shown are pHAGE6-EEK-b12a-ZsGr and control vector pHAGE6-EEK-luc-ZsGr. The HIV-1 5′ LTR, b12 mAb heavy chain variable region (b12VH), human IgA2 heavy chain constant region α1, α2, α3 (CHα1, CHα2, CHα3), picornavirus-derived self-cleaving 2A peptide sequences (F2A and T2A), b12 κ light chain variable and constant region (b12VL-CLκ), human immunoglobulin J chain (J), the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and 3′ LTR are indicated. For control vectors, the b12-IgA sequence was replaced by the luciferase gene in the same lentiviral vector. The human κ light chain promoter preceded by enhancers and matrix association regions is denoted as iEκ. The vector, pHAGE6-EEK-b12a-ZsGr contains a b12-IgA tri-cistronic cassette and a reporter gene ZsGreen (ZsGr) linked via IRES for simultaneous expression. (B) Secretion of human monoclonal antibody b12-IgA in human cells. Secreted human IgA in culture supernatant from 293T cells, which were transfected with either the lentiviral vector carrying b12-IgA or control vector (FUGW), were measured by human IgA ELISA at multiple time points. (C) CD10+CD19+ pro–B-cell development from HSPCs. Lentiviral transduction and in vitro human B lymphopoiesis culture were performed as previously described.22 CD34+ HSPCs transduced with either pHAGE6-EEK-luc-ZsGr (luc) or pHAGE6-EEK-b12a-ZsGr (b12-IgA) lentiviral constructs or left uninfected (none) cells were primed with IL-3, Flt3 ligand, thrombopoietin, SCF, and G-CSF for 5 days and then cocultured with MS5 stromal cells for indicated time points (C-E). Contour plots show CD10−CD19− cell population at 14 days (left, D14) and emerging CD10+CD19+ pro–B-cell population after 21 days (right, D21) of culture. (D) Increased surface IgM expression. Data shown are gated on CD19+ cells from day 14 (D14) or day 32 (D32) culture. (E) Transgene expression during B-cell lymphopoiesis. Transferred luciferase or b12-IgA gene expression was assessed with the fluorescence of coexpressed ZsGreen protein using flow cytometry at indicated time points; day 14 (D14), day 21 (D21), day 32 (D32), and day 49 (D49) of HSPCs culture in the presence of MS5 cells. (F) Development to B-cell blast from mature B cells by stimulation. After being cocultured with MS5 cells for 8 to 9 weeks, CD19+ B cells were isolated and stimulated as previously described.22 The 2 left panels of the contour plot show levels of IgM on CD19+ B cells before and after stimulation. The 2 right panels depict forward versus side scatter contour plots before and after stimulation. Data shown are gated on CD19+ cells.
Development of human B cells from HSPCs transduced with b12-IgA gene in vitro. (A) Schematic representation of the lentiviral constructs. Shown are pHAGE6-EEK-b12a-ZsGr and control vector pHAGE6-EEK-luc-ZsGr. The HIV-1 5′ LTR, b12 mAb heavy chain variable region (b12VH), human IgA2 heavy chain constant region α1, α2, α3 (CHα1, CHα2, CHα3), picornavirus-derived self-cleaving 2A peptide sequences (F2A and T2A), b12 κ light chain variable and constant region (b12VL-CLκ), human immunoglobulin J chain (J), the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and 3′ LTR are indicated. For control vectors, the b12-IgA sequence was replaced by the luciferase gene in the same lentiviral vector. The human κ light chain promoter preceded by enhancers and matrix association regions is denoted as iEκ. The vector, pHAGE6-EEK-b12a-ZsGr contains a b12-IgA tri-cistronic cassette and a reporter gene ZsGreen (ZsGr) linked via IRES for simultaneous expression. (B) Secretion of human monoclonal antibody b12-IgA in human cells. Secreted human IgA in culture supernatant from 293T cells, which were transfected with either the lentiviral vector carrying b12-IgA or control vector (FUGW), were measured by human IgA ELISA at multiple time points. (C) CD10+CD19+ pro–B-cell development from HSPCs. Lentiviral transduction and in vitro human B lymphopoiesis culture were performed as previously described.22 CD34+ HSPCs transduced with either pHAGE6-EEK-luc-ZsGr (luc) or pHAGE6-EEK-b12a-ZsGr (b12-IgA) lentiviral constructs or left uninfected (none) cells were primed with IL-3, Flt3 ligand, thrombopoietin, SCF, and G-CSF for 5 days and then cocultured with MS5 stromal cells for indicated time points (C-E). Contour plots show CD10−CD19− cell population at 14 days (left, D14) and emerging CD10+CD19+ pro–B-cell population after 21 days (right, D21) of culture. (D) Increased surface IgM expression. Data shown are gated on CD19+ cells from day 14 (D14) or day 32 (D32) culture. (E) Transgene expression during B-cell lymphopoiesis. Transferred luciferase or b12-IgA gene expression was assessed with the fluorescence of coexpressed ZsGreen protein using flow cytometry at indicated time points; day 14 (D14), day 21 (D21), day 32 (D32), and day 49 (D49) of HSPCs culture in the presence of MS5 cells. (F) Development to B-cell blast from mature B cells by stimulation. After being cocultured with MS5 cells for 8 to 9 weeks, CD19+ B cells were isolated and stimulated as previously described.22 The 2 left panels of the contour plot show levels of IgM on CD19+ B cells before and after stimulation. The 2 right panels depict forward versus side scatter contour plots before and after stimulation. Data shown are gated on CD19+ cells.
Generation of humanized mice expressing b12-IgA and mucosal targeting of transgene
To test for in vivo production of b12-IgA from transduced HSPCs, we turned to 2 humanized mouse models. The first and more complex was the hu-BLT mouse, which is known to support a stably reconstituted human immune system.30 We generated b12-IgA-transduced hu-BLT mice by cotransplanting human fetal CD34+ HSPCs infected with lentivirus vectors, autologous fetal liver, and thymic tissues (Figure 3A).
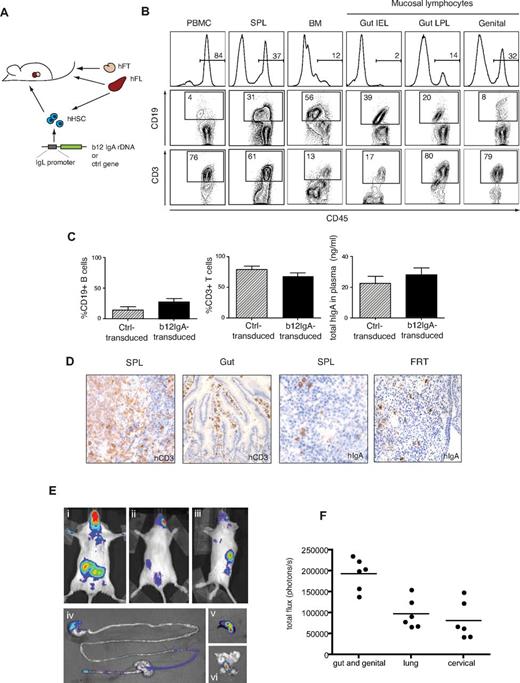
Generation of hu-BLT mice expressing b12-IgA through HSPC gene transfer. (A) A schematic diagram of the generation of hu-BLT mice transduced with human b12-IgA gene (hu-BLT-b12a mice). (B) Peripheral and mucosal reconstitution of a human immune system in hu-BLT-b12a mice. Flow cytometry shows human cell engraftment in various tissues isolated at 14 to 16 weeks after transplantation; PBMC indicates peripheral blood mononuclear cells; SPL, spleen; BM, bone marrow; Gut IEL, intestinal intraepithelial lymphocytes; Gut LPL, intestinal lamina propria lymphocytes; and genital, genital tract lymphocytes. (C) Percent engraftment of human B and T lymphocytes in periphery (Ctrl-transduced mice n = 7, b12-IgA-transduced mice n = 21) and the secretion of human IgA in the plasma (Ctrl-transduced mice, n = 5, b12-IgA-transduced mice, n = 12) of hu-BLT mice either transduced with b12-IgA gene or the control gene (mean ± SEM). (D) Immunohistochemical staining for human CD3 in spleen and small intestine tissues and human IgA-Producing cells in spleen and female reproductive tract (FRT) of hu-BLT-b12a mice. Samples were examined on an Olympus BX-51 microscope (20× objective lens) and photographed using a Spot Digital Camera. (E) Bioluminescence images of hu-BLT mice transduced with IgL chain promoter-driven luciferase transgene. The representative images of live animals displayed the distribution of human B-lymphocytes derived from transplanted human HSPCs that express transgenes; (Ei) ventral view of reference, (Eii) dorsal view of reference, and (Eiii) lateral view of reference. Strong bioluminescent signals from mucosal associated lymphoid tissues near gut, lung and genital tract area are seen in ventral view (Ei). Secondary lymphoid tissue (spleen) signals are seen in dorsal (Eii) and lateral view (Eiii). A luciferin-injected mouse was sacrificed after 5 minutes of incubation and dissected immediately to excise organs and tissues. The representative images of GI tract (Eiv), spleen (Ev), and genital tract (Evi) show tissue specificity of transgene expression. (F) Luminescent signals from hu-BLT mice described above at 18 weeks after transplantation. Signals recorded in photons/second were acquired from each indicated areas in the whole animal image.
Generation of hu-BLT mice expressing b12-IgA through HSPC gene transfer. (A) A schematic diagram of the generation of hu-BLT mice transduced with human b12-IgA gene (hu-BLT-b12a mice). (B) Peripheral and mucosal reconstitution of a human immune system in hu-BLT-b12a mice. Flow cytometry shows human cell engraftment in various tissues isolated at 14 to 16 weeks after transplantation; PBMC indicates peripheral blood mononuclear cells; SPL, spleen; BM, bone marrow; Gut IEL, intestinal intraepithelial lymphocytes; Gut LPL, intestinal lamina propria lymphocytes; and genital, genital tract lymphocytes. (C) Percent engraftment of human B and T lymphocytes in periphery (Ctrl-transduced mice n = 7, b12-IgA-transduced mice n = 21) and the secretion of human IgA in the plasma (Ctrl-transduced mice, n = 5, b12-IgA-transduced mice, n = 12) of hu-BLT mice either transduced with b12-IgA gene or the control gene (mean ± SEM). (D) Immunohistochemical staining for human CD3 in spleen and small intestine tissues and human IgA-Producing cells in spleen and female reproductive tract (FRT) of hu-BLT-b12a mice. Samples were examined on an Olympus BX-51 microscope (20× objective lens) and photographed using a Spot Digital Camera. (E) Bioluminescence images of hu-BLT mice transduced with IgL chain promoter-driven luciferase transgene. The representative images of live animals displayed the distribution of human B-lymphocytes derived from transplanted human HSPCs that express transgenes; (Ei) ventral view of reference, (Eii) dorsal view of reference, and (Eiii) lateral view of reference. Strong bioluminescent signals from mucosal associated lymphoid tissues near gut, lung and genital tract area are seen in ventral view (Ei). Secondary lymphoid tissue (spleen) signals are seen in dorsal (Eii) and lateral view (Eiii). A luciferin-injected mouse was sacrificed after 5 minutes of incubation and dissected immediately to excise organs and tissues. The representative images of GI tract (Eiv), spleen (Ev), and genital tract (Evi) show tissue specificity of transgene expression. (F) Luminescent signals from hu-BLT mice described above at 18 weeks after transplantation. Signals recorded in photons/second were acquired from each indicated areas in the whole animal image.
Human immune cells derived from transplanted HSPCs reconstituted the immune system in peripheral blood, lymphoid organs, and mucosal sites such as the GI tract or genital tract (Figure 3B). Cells transduced with the gene for b12-IgA showed engraftment levels comparable with those transduced with the control gene (Figure 3C, supplemental Figure 3). Stable expression of a mucosal homing receptor, α4β7 integrin, which mediates gut and genital tropism, in the B and T lymphocytes of the gut and genital mucosa indicated an efficient reconstitution of the mucosal immune system in the b12-IgA transduced animals (supplemental Figure 4). Immunohistologic analysis confirmed human T cells and IgA-producing B cells were located in the spleen and mucosal tissues, such as the gut and female reproductive tract (Figure 3D).
To visualize transgene expression by bioluminescence imaging, we used the animals harboring a luciferase gene with the same IgL promoter. Luciferase expression was detected by live animal imaging in multiple areas of transduced hu-BLT mice; the lower abdomen; the upper left side, at the site of the spleen; the lower center area, close to the caudal region (Figure 3Ei-iii). Most of the animals also showed bioluminescence in the cervicofacial region and upper thoracic region (Figure 3Ei). Ex vivo bioluminescence imaging of selected organs revealed that the transgene was expressed throughout the GI tract, spleen and genital tract (Figure 3Eiv-vi). The strongest signal appeared in the area encompassing gut and genital tract in almost all mice examined (Figure 3F). This bioluminescence analysis demonstrated that gene transfer through HSPCs using the IgL promoter induces specific transgene expression predominantly targeted to the lymphoid and mucosal tissues in vivo. The luciferase activity derived from transgene expression was steadily detected in the mice until the end of the study (from 24-30 weeks after transplantation), indicating stable expression of the transgene in cells derived from HSPC transplants.
B cell and plasma cell–specific expression of b12-IgA in mucosal and lymphoid tissues of humanized mice
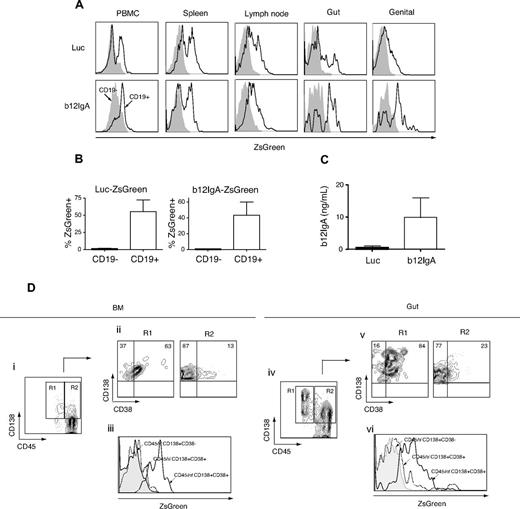
We also examined the cell type specificity of transgene expression in lymphoid and mucosal tissues. Whereas little to no transgene expression was detected in the non–B-cell population, expression was evident in the mature B cells of gut and genital tract lymphocytes as well as PBMCs and lymphocytes from lymphoid organs (Figure 4A-B). A substantial proportion of lymphocytes homing to the mucosal tissues were expressing the transgene, confirming the data obtained from bioluminescence imaging. These results indicate that the IgL promoter-driven, HSPC-based gene transfer should induce B cell–specific expression of a b12-IgA transgene, predominantly targeted to mucosal tissues and lymphoid organs. Human b12-IgA antibody was detected in plasma in b12-IgA–transduced mice (Figure 4C). Secreted b12-IgA antibody was also detected in mucosal secretions such as the bronchoalveolar lavage (BAL), saliva, and intestinal lavage collected from selected mice (supplemental Table 1).
b12-IgA transgene expression in B cells and plasma cells in mucosal and lymphoid tissues. (A) Human B cell (CD45+CD19+)–specific expression of transgene in hu-BLT mice. Gut-homing lymphocytes were isolated from the epithelia and lamina propria of the small and large intestine and genital tract-homing lymphocytes were isolated from the entire genital tract except the testis or ovary. Flow cytometry of the ZsGreen reporter gene expression of the lymphocytes isolated from PBMC, spleen, lymph node, gut and genital tract. The ZsGreen fluorescence of CD19− cells (filled histogram) and CD19+ (solid lines) was analyzed after gating on CD45+ human leukocytes. (B) Percent transgene expression of CD19− (black) and CD19+ (white) cells from peripheral blood of hu-BLT mice transduced with Luc-ZsGreen vector or b12-IgA-ZsGreen vector (n = 4-5, mean ± SEM). (C) Enzyme-linked immunosorbent assay of the secreted b12-IgA in plasma of hu-BLT mice transduced with Luc-ZsGreen vector (black) or b12-IgA-ZsGreen vector (white) at 10 weeks posttransplantation of human HSPCs (n = 6-8, mean ± SEM). (D) Plasma cell development from transplanted human HSPCs in hu-BLT mice transduced with b12-IgA-ZsGreen and transgene expression in long-lived plasma cells. Flow cytometry of bone marrow mononuclear cells (Di-iii) and gut lymphocytes (Div-Dvi) isolated from 22 to 24 week posttransplanted hu-BLT mice, with gating on CD45+ cells. The contour plots (Di,iv) show CD138+CD45int plasma cells (R1) and CD138+CD45hi (R2) plasma cells. R1 and R2 cell populations were then separately plotted by CD38 and CD138 expression (Dii,v). Histograms (Diii,vi) display reporter gene ZsGreen expression in CD45hiCD138+CD38− cells, CD45hiCD138+CD38+ early plasma cells and CD45intCD138+CD38+ mature plasma cells.
b12-IgA transgene expression in B cells and plasma cells in mucosal and lymphoid tissues. (A) Human B cell (CD45+CD19+)–specific expression of transgene in hu-BLT mice. Gut-homing lymphocytes were isolated from the epithelia and lamina propria of the small and large intestine and genital tract-homing lymphocytes were isolated from the entire genital tract except the testis or ovary. Flow cytometry of the ZsGreen reporter gene expression of the lymphocytes isolated from PBMC, spleen, lymph node, gut and genital tract. The ZsGreen fluorescence of CD19− cells (filled histogram) and CD19+ (solid lines) was analyzed after gating on CD45+ human leukocytes. (B) Percent transgene expression of CD19− (black) and CD19+ (white) cells from peripheral blood of hu-BLT mice transduced with Luc-ZsGreen vector or b12-IgA-ZsGreen vector (n = 4-5, mean ± SEM). (C) Enzyme-linked immunosorbent assay of the secreted b12-IgA in plasma of hu-BLT mice transduced with Luc-ZsGreen vector (black) or b12-IgA-ZsGreen vector (white) at 10 weeks posttransplantation of human HSPCs (n = 6-8, mean ± SEM). (D) Plasma cell development from transplanted human HSPCs in hu-BLT mice transduced with b12-IgA-ZsGreen and transgene expression in long-lived plasma cells. Flow cytometry of bone marrow mononuclear cells (Di-iii) and gut lymphocytes (Div-Dvi) isolated from 22 to 24 week posttransplanted hu-BLT mice, with gating on CD45+ cells. The contour plots (Di,iv) show CD138+CD45int plasma cells (R1) and CD138+CD45hi (R2) plasma cells. R1 and R2 cell populations were then separately plotted by CD38 and CD138 expression (Dii,v). Histograms (Diii,vi) display reporter gene ZsGreen expression in CD45hiCD138+CD38− cells, CD45hiCD138+CD38+ early plasma cells and CD45intCD138+CD38+ mature plasma cells.
Plasma cells are the terminally differentiated antibody-secreting B cells and play crucial roles in effective humoral immune responses, particularly in the mucosal compartments. More than 80% of human plasma cells31 are located in the mucosal lamina propria of the GI tract, genital tract, and respiratory tracts.32,33 To investigate terminal differentiation to plasma cells from transplanted HSPCs in the b12-IgA–transduced hu-BLT mice, bone marrow mononuclear cells (BMMCs) and gut-homing lymphocytes were isolated and analyzed for the expression of plasma cell–specific markers (CD138, CD38) by flow cytometry.
CD45 expression decreases during plasma cell differentiation, and this decrease is associated with an increasing maturity and an enhanced life-span.34,35 Analysis of the BMMCs and gut-homing lymphocytes revealed 2 distinct populations of these cells; more mature CD45intCD138+ (R1) and less differentiated CD45hiCD138+ (R2) cells (Figure 4Di-iv). The 2 populations were then further analyzed for the expression of another plasma cell marker CD38 (Figure 4Dii-v). Importantly, transgene expression was higher in CD45intCD138+CD38+ plasma cells, which are the most mature, longest-lived plasma cells, than in CD45hiCD138+CD38+ early plasma cells and in less differentiated CD45hiCD138+CD38− cells (Figure 4Diii-vi). These results indicate that our strategy can support a long-term, stable production of effector molecules delivered by HSPC gene therapy in the periphery and mucosa of transplanted animals.
Inhibition of HIV-1 mucosal transmission in b12-IgA expressing hu-BLT mice
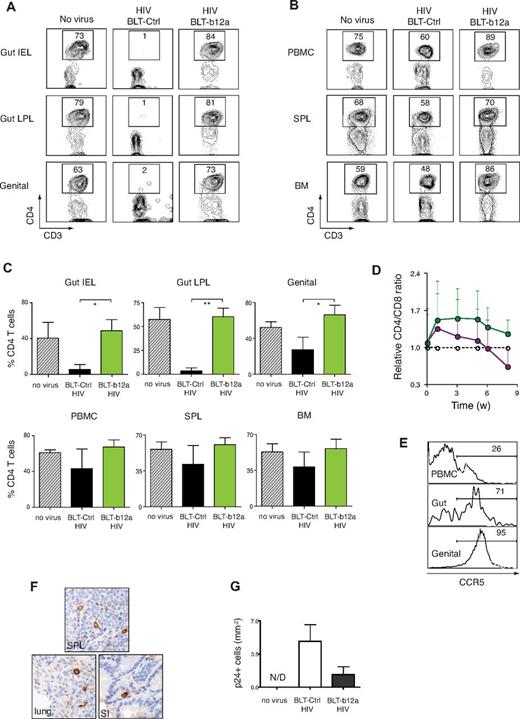
To test whether the HSPC-based gene transfer introducing an IgA form of bNAb could inhibit HIV-1 mucosal transmission in treated animals, we administered HIV-1 intravaginally to b12-IgA or control vector-transduced hu-BLT mice. The human cell engraftment and CD4+ T cell numbers in the mice before viral challenge were in a comparable range (supplemental Figure 5). Acute loss of the mucosal CD4+ T cells is one of the hallmarks of HIV infection. After HIV-1 challenge, CD4+ T cells were almost completely depleted from intestinal IEL and LPL and markedly reduced in the genital tract of the control gene-transduced hu-BLT mice. However, mice expressing the b12-IgA were entirely protected from the HIV-1–induced massive depletion of CD4+ T cells in the gut and genital tract (Figure 5A-C). CD4+ T cells populations in unprotected mice were reduced in the PBMC, spleen, and bone marrow but to a lesser extent than seen in the mucosa. Mice expressing b12-IgA maintained their proportion of CD4+ T cells in lymphoid tissues and peripheral blood at the level of unchallenged mice (Figure 5B-C).
Prevention of mucosal transmission of HIV-1 in hu-BLT mice transduced with b12-IgA. Humanized-BLT mice were challenged with R5 tropic HIV-1 (JR-CSF) through intravaginal route at 14 to 20 weeks after transplantation or left unchallenged (no virus). Peripheral blood of the mice was collected periodically. Mice were killed after 8 to 10 weeks of HIV-1 challenge and lymphocytes from various tissues were isolated and analyzed by flow cytometry. (A) Protection of mucosal CD4+ T cells in hu-BLT-b12a mice from HIV-1 infection. Flow cytometry of mucosal lymphocytes isolated from intestinal intraepithelium (gut IEL), intestinal lamina propria (gut LPL) and female genital tract (genital) of hu-BLT mice that are transduced with b12-IgA gene (BLT-b12a) or with control gene (BLT-Ctrl). (B) Proportion of CD4+ T cells of primary (BM indicates bone marrow) or secondary (SPL indicates spleen) lymphoid organs and periphery. Cells were pregated on CD45+CD3+ cells (A-B). (C) Percent CD4+ T cells in CD45+CD3+ human T cells in the tissues of hu-BLT mice after HIV-1 infection. Data are mean ± SEM (n = 3-5, *P ≤ .05, **P ≤ .01). (D) Changes of CD4/CD8 ratio in PBMCs of hu-BLT mice after HIV-1 mucosal challenge. Data are mean ± SEM at each time points (weeks after challenge) open circle: no challenge; purple circle: HIV-1 challenge in control gene-transduced hu-BLT mice; green circle: HIV-1 challenge in b12-IgA-transduced hu-BLT mice. Unpaired t test showed the b12-IgA transduced group had significantly higher CD4:CD8 ratios than the control vector transduced group after HIV challenge. (n = 3-6, P < .05, P = .0148) All data points in each group regardless of time were included in the t test. (E) Differential expression of CCR5, the HIV-1 coreceptor level in human CD4+ T cells in hu-BLT mice. Histograms show the proportion of CCR5+CD4+ T cells in PBMC, gut lymphocyte, genital tract lymphocytes of hu-BLT mouse model. (F) Immunohistochemical staining of HIV-1 p24 protein in indicated tissues of hu-BLT mice after HIV-1 mucosal challenge. (SPL: spleen, SI: small intestine) Samples were examined on an Olympus BX-51 microscope (40× objective lens) and photographed using a Spot Digital Camera. (G) P24+ cells were determined by counting immunohistochemically stained cells from genital and intestinal tract tissue sections of hu-BLT mice. (n = 3-5, mean ± SEM).
Prevention of mucosal transmission of HIV-1 in hu-BLT mice transduced with b12-IgA. Humanized-BLT mice were challenged with R5 tropic HIV-1 (JR-CSF) through intravaginal route at 14 to 20 weeks after transplantation or left unchallenged (no virus). Peripheral blood of the mice was collected periodically. Mice were killed after 8 to 10 weeks of HIV-1 challenge and lymphocytes from various tissues were isolated and analyzed by flow cytometry. (A) Protection of mucosal CD4+ T cells in hu-BLT-b12a mice from HIV-1 infection. Flow cytometry of mucosal lymphocytes isolated from intestinal intraepithelium (gut IEL), intestinal lamina propria (gut LPL) and female genital tract (genital) of hu-BLT mice that are transduced with b12-IgA gene (BLT-b12a) or with control gene (BLT-Ctrl). (B) Proportion of CD4+ T cells of primary (BM indicates bone marrow) or secondary (SPL indicates spleen) lymphoid organs and periphery. Cells were pregated on CD45+CD3+ cells (A-B). (C) Percent CD4+ T cells in CD45+CD3+ human T cells in the tissues of hu-BLT mice after HIV-1 infection. Data are mean ± SEM (n = 3-5, *P ≤ .05, **P ≤ .01). (D) Changes of CD4/CD8 ratio in PBMCs of hu-BLT mice after HIV-1 mucosal challenge. Data are mean ± SEM at each time points (weeks after challenge) open circle: no challenge; purple circle: HIV-1 challenge in control gene-transduced hu-BLT mice; green circle: HIV-1 challenge in b12-IgA-transduced hu-BLT mice. Unpaired t test showed the b12-IgA transduced group had significantly higher CD4:CD8 ratios than the control vector transduced group after HIV challenge. (n = 3-6, P < .05, P = .0148) All data points in each group regardless of time were included in the t test. (E) Differential expression of CCR5, the HIV-1 coreceptor level in human CD4+ T cells in hu-BLT mice. Histograms show the proportion of CCR5+CD4+ T cells in PBMC, gut lymphocyte, genital tract lymphocytes of hu-BLT mouse model. (F) Immunohistochemical staining of HIV-1 p24 protein in indicated tissues of hu-BLT mice after HIV-1 mucosal challenge. (SPL: spleen, SI: small intestine) Samples were examined on an Olympus BX-51 microscope (40× objective lens) and photographed using a Spot Digital Camera. (G) P24+ cells were determined by counting immunohistochemically stained cells from genital and intestinal tract tissue sections of hu-BLT mice. (n = 3-5, mean ± SEM).
We also examined the changes of CD4+ T cell population during the course of HIV infection in PBMCs. The CD4/CD8 T-cell ratio in PBMCs gradually decreased during the acute infection, resulting in an approximately 30% loss of peripheral CD4+ T cells compared with the unchallenged mice. However, the ratio was sustained in the mice expressing b12-IgA (Figure 5D), indicating that peripheral T-cell loss mediated by systemic HIV-1 spread was inhibited in the b12-IgA expressing mice. Immediate mucosal CD4+ T cell depletion and gradual peripheral CD4+ T cell loss served as a surrogate marker of disease progression. The differential severity of CD4+ T-cell depletion in the peripheral and mucosal compartments after HIV-1 challenge was probably because of a differential expression of CCR5 in peripheral CD4+ T cells compared with mucosal T cells. In this animal model, up to 95% of CD4+ T cells in genital tract and gut expressed CCR5, whereas only approximately 26% of CD4+ T cells expressed CCR5 in the periphery (Figure 5E). Thus, CD4+ T-cell loss was more drastic in the mucosal compartment where the target T cells (CCR5+CD4+) for R5-tropic HIV were most abundant compared with peripheral blood, where target cells were limited. Because the study period in which we examined the T-cell loss mainly represents an acute infection, a complete depletion of peripheral CD4+ T cell was not observed in our experimental conditions. In fact, the profile of peripheral CD4+ T-cell reduction in our study is similar to that in a typical acute infection with HIV-1 in humans, where there is an approximately 35% loss of peripheral CD4+ T cells after an acute infection.36 HIV-1 virus-producing cells were detected by immunohistochemical staining of the viral capsid protein p24 in tissues including the lung, intestine, and spleen (Figure 5F). The quantitative analysis of immunohistochemistry revealed an approximately 70% reduction in the number of HIV-producing cells in the tissues of the mice expressing b12-IgA (Figure 5G).
We confirmed the prophylactic effect of anti-HIV IgA in mucosal protection in another humanized mouse model, the human immune system (HIS) mouse model that is generated by injecting human cord blood CD34+ HSPCs intrahepatically into neonatal Rag2−/−IL-2γC−/− mice.37 To produce humanized mice expressing b12-IgA antibody, the HSPCs were transduced with a lentivirus encoding the b12-IgA gene. Human T-cell and B-cell populations were reconstituted as observed in the hu-BLT mice (supplemental Table 2). When challenged with HIV-1JR-CSF intravaginally, the control HIS mice exhibited severe CD4+ T cell depletion at mucosal sites. However, the HIS mice transduced with the b12-IgA gene maintained their proportion of CD4+ T cells in the GI and genital tract, similarly to what was observed in the hu-BLT mouse model. The CD4+ T cell loss after HIV-1 mucosal challenge was also observed in the PBMC and lymphoid organs of the control animals, whereas such loss was not observed in the HIS mice transduced with the b12-IgA (supplemental Figure 6A). The peripheral CD4/CD8 T-cell ratio gradually decreased over time after HIV infection of HIS mice, whereas the ratio gradually increased in the b12-IgA transduced mice (supplemental Figure 6B). Virtually no HIV-producing p24+ cells were detected in mice expressing b12-IgA (supplemental Figure 6C). These results demonstrate that b12-IgA gene transfer provides protection from HIV mucosal transmission to HIS mice.
Discussion
In this study, we demonstrated that anti-HIV IgA has a more potent prophylactic effect than its IgG counterpart on mucosal transmission of HIV in vivo. Furthermore, we provided anti-HIV IgA to humanized mice through HSPC-mediated gene transfer in a cell-specific manner. We showed that engineered HSPC transplants developed into B cells, further differentiated into plasma cells, and produced b12-IgA in blood and mucosal tissues in vivo. In this way, we found that the class-switched IgA form of b12 Ab produced in vivo could inhibit HIV mucosal transmission in 2 different humanized mouse models.
It is notable that the very low steady-state level of endogenously expressed b12-IgA (average 10 ng/mL in plasma and 4-7 ng/mL in mucosal secretions) was able to inhibit the loss of mucosal CD4 cells caused by HIV infection in transduced humanized mice. Our results from the passive transfer of IgA and IgG in Figure 1 demonstrated that IgA inhibited HIV infection more efficiently than IgG after mucosal challenge. Two-thirds of mice intravenously administered with b12-IgA were protected from HIV-induced CD4 T-cell depletion at the plasma b12-IgA antibody concentrations ranged from 5 to 10 μg/mL. Although the plasma b12-IgA antibody concentrations that provided protection in passive transfer were higher than those in b12-IgA–transduced BLT mice, the corresponding Ab concentrations detected in vaginal secretions were comparable in both experiments (< 10 ng/mL). This may be because of the difference between single-dose infusion and steady-state endogenous expression of Ab.
The ability of IgA to provide protection at low concentration may be explained by unique functions of pIgA in the mucosal environment, because the original b12-IgG and the monomeric b12-IgA showed almost equivalent activities for in vitro neutralization assay (Figure 1A and Mantis et al26 ) or for protection against HIV challenge in vivo (Figure 1). PIgA is selectively transported from the lamina propria to the mucosal lumen by transcytosis mediated by polymeric immunoglobulin receptor (pIgR) expressed on epithelial cells. The transported sIgA consists of pIgA bound to a proteolytically cleaved fragment of pIgR called secretory component. SIgA is able to mediate mucosal protection through multiple mechanisms, such as immune exclusion, intracellular neutralization, and antigen excretion as well as by neutralization of free virus.38
Previous studies have shown that monoclonal Abs class-switched to pIgA or IgM, but not the original IgG, were able to inhibit transepithelial entry of HIV-1 in vitro using an intestinal epithelial cell line.2 The intracellular neutralization of HIV-1 occurred within epithelial cells by HIV-specific dimeric IgA39,40 and IgA-mediated excretion of HIV-1 has been shown in vitro using polarized epithelium.3 These in vitro studies demonstrated the unique functions of SIgA in epithelial cells that IgG is not able to accomplish. Our study directly demonstrates the potency of IgA against virus infection in the mucosal environment in vivo. The unique ability of sIgA may provide the explanation of how very low concentrations of sIgA are able to block virus infection at the mucosal portal of entry, providing a first line of defense.
Moreover, sIgA achieves immune protection without activating the classic complement pathway, whereas IgG is a strong activator of the complement cascade. Although complement activation generally contributes to neutralization of a pathogen in concert with NAb, the contribution of complement to HIV protection does not appear to be critical. A b12-IgG variant defective in complement binding blocked SHIV infection in macaques as effectively as wild-type b12.41 Moreover, recent data have indicated that complement activation may rather enhance HIV infection at later stages through the binding of HIV-complement immune complexes to complement receptor positive cells.42 Serum IgA also contributes to the control of HIV replication by triggering antibody-dependent cellular cytotoxicity (ADCC) through myeloid IgA receptor FcαRI.43
The involvement of IgG and FcγR-mediated ADCC to mucosal protection of HIV infection appears to be more complicated. Although a b12-IgG variant defective in FcγR binding showed reduced protective activity in vivo,41 a b12-IgG variant with increased affinity to FcγR and enhanced Fc-dependent antiviral activities in vitro did not enhance protection in vivo.44 The inability of ADCC-mediating but nonneutralizing mAbs to protect against HIV mucosal infection underscores the importance of neutralizing antibody rather than FcγR-mediated ADCC in HIV protection.45
In our intravaginal challenge of HIV-1 using a CCR5-tropic strain JR-CSF, we observed a profound mucosal CD4+ T-cell depletion in the unprotected humanized mice. Drastic depletion of mucosal CD4+ T cells has been observed in HIV infection of humans as well as SIV infection of monkeys.46 Because of the expression of CCR5 and the abundance of activated T cells, gut CD4+ T cells are highly susceptible to HIV. Furthermore, integrin α4β7, a homing receptor that mediates recruitment to the gut and genital tract, was recently identified as an attachment receptor for HIV that facilitates virus entry.47 After the structural integrity of the gut mucosa is destroyed as a result of HIV infection and massive depletion of CD4+ T cells, microbial translocation from the gut occurs resulting in nonspecific immune activation and accumulation of activated T cells, which creates new targets for HIV. This may be the reason that gut CD4+ T cell depletion preceded peripheral CD4+ T cells decline in our study. Our results suggest that neutralizing IgA antibody against HIV is able to inhibit systemic spread by protecting the preferential targets of HIV in gut mucosa.
HIV viral protein p24 was also detected in the mucosal and lymphoid tissues sections of the BLT mice. However, the viral load in the mouse plasma measured by HIV viral RNAs was not greater than the detection limit of our method in these mice, although viral RNA was detected in NSG-hu mice used in passive transfer experiment in Figure 1. This might be partly because peripheral T cells in NSG-hu mice were preactivated in vitro before transplantation and more susceptible to HIV-1 infection, whereas the majority of peripheral T cells in BLT mice were not activated and CCR5-negative (Figure 5E). It is also possible that the time points we measured were not at the peak of viral replication during an acute infection in this mouse model.
Our results demonstrate that engineered humoral immunity with a potent neutralizing anti-HIV antibody using HSPCs can effectively reduce disease progression and this approach will be even more beneficial if it is applied in combination with other recently discovered potent broadly neutralizing antibodies.6-8 We have also shown the pivotal function of class-switched IgA form of antibody and its mucosal targeting in the protection of HIV transmission in vivo. Thus, our study may provide new insights into the reprogramming of human immunity using genetically engineered HSPCs to provide the therapeutic potential of engineered IgA for the treatment of various infectious diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Nabel (National Institute of Allergy and Infectious Diseases) for the b12-IgG plasmid; B. Corthésy (University State Hospital [CHUV], Switzerland) for human IgA2 plasmids; A. Balazs (California Institute of Technology) for lentiviral vectors; and P. Bjorkman and A. West (both from California Institute of Technology) for the purified proteins.
This work was supported by the Bill & Melinda Gates Foundation, National Institutes of Health (NIH) grant HHSN266200500035C, and NIH career development award 5K08CA133521 (D.S.R.).
National Institutes of Health
Authorship
Contribution: E.M.H. and D.B. conceived the study with assistance from L.Y.; E.M.H. designed the experiments; E.M.H. and S.N.P. performed experiments and analyzed the data; S.S. performed transplantation to generate hu-BLT mice with direction from D.S.A.; D.S.R. assisted with analysis of immunohistochemistry; P.N.P.G. performed in vitro neutralization assay of antibodies; and E.M.H. and D.B. wrote the paper with contributions from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.S.R. is Department of Pathology and Laboratory Medicine, David Geffen School of Medicine and Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA.
Correspondence: David Baltimore, or LiLi Yang, Division of Biology, California Institute of Technology, 1200 E California Blvd, Pasadena, CA, 91125; e-mail: baltimo@caltech.edu or liyang@calteh.edu.

![Figure 1. HIV-1 infection in humanized mice passively infused with b12-IgA or b12-IgG isotype. (A) Neutralization activity of anti-HIV human mAb b12-IgA. Reporter cell line TZM-bl cells that express CD4, CXCR4, CCR5, and a Tat-responsive reporter gene for luciferase were infected with 200 TCID50 of replication-defective pseudovirus containing Env (SF162.LS) in the presence of various concentrations of anti-HIV monoclonal antibodies. Neutralization activity was measured by the reduction in luciferase reporter gene expression after a single round of pseudovirus infection in TZM-bl cells in triplicate. b12-IgG1 and the recombinant b12-IgA2 were compared at indicated concentrations. (B) Antibody level in circulation after passive transfer. NSG-hu mice were injected intravenously with various concentrations of purified b12-IgA2 (pIgA: mIgA = 1:1, mass ratio) or b12-IgG1 and the blood was collected after 4 hours. The plasma antibody concentrations were measured using ELISA. (n = 4-6, mean ± SEM). (C-D) Concentrations of b12 antibodies in plasma (C) or genital secretions (D) at the time of challenge. NSG-hu mice were injected intravenously with either 200 μg of b12-IgA2 or 20 μg of b12-IgG1 per mouse. Blood and genital secretions were collected after 4 hours. (E-I) Peripheral CD4+ T cell loss after HIV-1 challenge in NSG-hu mice injected with different b12 antibody isotypes; (E) purified human IgG/κ control antibody (hIgG/κ); (F) b12-IgA2 includes both monomeric and polymeric IgA as described in (B), (b12IgA2 [M+P]); (G) b12-IgG1; (H) b12-IgA2 monomer only (b12IgA2 [M]). Mice were challenged intravaginally with HIV-1JR-CSF. (I) Average percent CD4+ T cells in CD3 T cells in PBMCs at each time points (n = 5-9). Statistical analysis was performed using unpaired t test. (*P = .0313 and ***P < .0001 between IgA [M+P] +HIV and control Ab [hIgG/κ] + HIV). (J) Plasma viral loads in HIV infected NSG-hu mice. HIV viral RNA was measured by Abbott HIV viral load test. Dotted line indicates the detection limit. (N/D indicates not detected; n = 4, indicated P values are from unpaired t test between Ab-treated groups with HIV and hIgG/κ-treated groups with HIV.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/23/10.1182_blood-2012-04-422303/4/m_zh89991299780001.jpeg?Expires=1769093598&Signature=qKsFY9Fm0feoi~XEF-uqSqW-ipeZtSRIPrmJn85Efucuz6weX7IsKOO5VnPt5yM4E3HRwgt48qGVKczEsq-Y~qTfJu5BXHImz4jAHoq40v2eo9zu84xkPk5nB5ccofTpQQU5HhZrQP~K5SRH9HsATCfq62c0vsC8FJbSneXdfvPshTOUAZYv4gkcS8hxzFSeTR~E7TRcfCocGClkKKfF8Hy-TBcS4Aev60r6vuma9uRAqNKisRm2OhPzTEfe2mIM1gIsDekLm7AwsabcInI6xWyFeMgD991Q6G20GcrA9D96M4UY6qTm-cONgDUfXJFK-CrdXdIYnANG5bwWl2qxXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal