Abstract
Gene therapy may provide a cure for hemophilia and overcome the limitations of protein replacement therapy. Increasing the potency of gene transfer vectors may allow improvement of their therapeutic index, as lower doses can be administered to achieve therapeutic benefit, reducing toxicity of in vivo administration. Here we generated codon-usage optimized and hyperfunctional factor IX (FIX) transgenes carrying an R338L amino acid substitution (FIX Padua), previously associated with clotting hyperactivity and thrombophilia. We delivered these transgenes to hemophilia B mice by hepatocyte-targeted integration-competent and -defective lentiviral vectors. The hyperfunctional FIX transgenes increased FIX activity reconstituted in the plasma without detectable adverse effects, allowing correction of the disease phenotype at lower vector doses and resulting in improved hemostasis in vivo. The combined effect of codon optimization with the hyperactivating FIX-R338L mutation resulted in a robust 15-fold gain in potency and therefore provides a promising strategy to improve the efficacy, feasibility, and safety of hemophilia gene therapy.
Introduction
Gene therapy may provide a cure for hemophilia and overcome the limitations of protein replacement therapy.1,2 The therapeutic potential of gene therapy for hemophilia B was recently established in a clinical trial using adeno-associated viral vectors,3 yielding sustained therapeutic factor IX (FIX) expression levels. Notwithstanding these encouraging results, further improvements are needed to enhance efficacy and to overcome the host immune response.
We and others have shown that liver-directed gene transfer by lentiviral vectors (LVs) can correct the disease phenotype in hemophilia B and A mice.4-6 LVs may offer some advantages compared with adeno-associated viral vectors, especially considering the capacity to accommodate larger inserts and the rare preexisting host immunity to the virus. Transgene expression is targeted to hepatocytes by a synthetic hepatocyte-specific promoter. Residual transgene expression in antigen-presenting cells of liver and spleen is abolished by including in the vector target sequences for the hematopoietic-specific microRNA 142, which targets for degradation any expressed transgene mRNA in this cell lineage.4,7 This resulted in induction of specific tolerance to the transgene product, including FIX.8,9 Nevertheless, to translate LV gene therapy for hemophilia to the clinic, the safety concerns associated with administering large doses of an integrating vector to the liver and the need for manufacturing large amounts of clinical-grade vector must be addressed.
Increasing the potency (efficacy per dose) of gene transfer vectors is crucial toward achieving these goals. It would allow using lower doses to obtain therapeutic benefit, thus reducing potential toxicities and immune activation associated with in vivo administration, easing manufacturing needs, and alleviating long-term safety concerns associated with genomic integration. One way to increase potency is to engineer the transgene sequence itself to maximize expression and biologic activity per vector copy. Here we show that FIX transgenes, optimized for codon usage and carrying an R338L amino acid substitution associated with clotting hyperactivity and thrombophilia,10 increase the efficacy of gene therapy up to 15-fold in hemophilia B mice, without detectable adverse effects, substantially reducing the dose requirement for reaching therapeutic efficacy and thus facilitating future scale up and its clinical translation.
Methods
Additional information can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The codon usage optimization was carried out using proprietary algorithms (BaseClear). The R338L mutation was introduced using QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies). Lentiviral vectors were produced and characterized as described.9 Adult hemophilia B mice11 were injected intravenously at the indicated vector doses. D-dimer and FIX antigen levels were determined by ELISA and FIX activity with activated partial thromboplastin time or a chromogenic assay (Hyphen Biomed). Tail-clipping assay was performed as described.12 All animal experiments were approved by the respective Animal Care and Use Committees.
Results and discussion
We cloned a canine codon-optimized FIX (co-cFIX) cDNA, or its wild-type (WT) counterpart (cFIX), into miR-142-regulated LVs under the transcriptional control of the hepatocyte-specific ET promoter (supplemental Figure 1). We compared the potency of the 2 transgenes in hemophilia B mice (5 or 10 × 108 transducing units [TU]/mouse). FIX activity was 3.4- and 2.2-fold higher for the co-cFIX than its WT counterpart in both dose cohorts (Figure 1A-B). Vector genome content was similar in the liver of treated animals receiving matched doses of LVs. FIX inhibitors were undetectable by Bethesda assay in all treated mice (not shown). These data show that a co-cFIX transgene can significantly increase expression, without any evident impairment of specific enzyme activity or increased immunogenicity.13
Evaluating codon-optimized and hyperfunctional FIX transgenes by LV delivery in hemophilic mice. Mice were intravenously administered matched doses (in transducing units, as measured on 293T cells) of LVs expressing the indicated FIX transgene. FIX expression and clotting activity were measured by ELISA (A,C,E) and activated partial thromboplastin time (B,D,F), respectively, on plasma samples collected at the indicated times after LV administration. Vector copies per diploid genome (vector copy number [VCN]) were measured at the end of the experiments in liver genomic DNA. (A-B) A total of 5 × 108 TU (filled line) of ET.cFIX.142T (squares, n = 4; VCN 1.3 ± 0.1) or ET.co-cFIX.142T (triangles, n = 4; VCN 0.9 ± 0.2); 1 × 109 TU (dashed line) of ET.cFIX.142T (squares, n = 4; VCN 1.6 ± 0.3) or ET.co-cFIX.142T (triangles, n = 4; VCN 1.4 ± 0.4). (C-D) A total of 7 × 108 TU of ET.cFIX.142T (squares, n = 4; VCN 1.1 ± 0.2) or ET.cFIXR338L.142T (diamonds, n = 6; VCN 1.3 ± 0.3) or ET.co-cFIXR338L.142T (triangles, n = 7; VCN 2.2 ± 0.2). (E-F) A total of 2.5 × 108 TU (black line, n = 3; VCN 1.4 ± 0.2) or 1.25 × 108 TU (gray line, n = 3; VCN 0.6 ± 0.1) of ET.co-cFIXR338L.142T. Data are mean ± SEM. *pGLOBAL < .05 (nonparametric combination statistics). ***pGLOBAL < .001 (nonparametric combination statistics). (G) Tail-clipping assay on hemophilia B mice treated with 2.5 × 108 TU of ET.co-cFIX.142T (n = 3) or ET.co-cFIXR338L (n = 3) as indicated. Blood loss (mean ± SEM) was determined by measuring the absorbance at 575 nm of hemoglobin content in the saline solution in which the tail was placed (black bars, left axis); cFIX activity (white bars, right axis). WT (n = 5) and untreated hemophilia B (HemoB) mice (n = 5) were used as controls. (H-I) FIX expression and clotting activity were measured by ELISA and chromogenic FIX assay, respectively, on plasma samples collected at the indicated times after 109 TU (n = 4) or 2 × 109 TU (n = 3) of ET.co-hFIXR338L.142T LV administration as indicated. Data are mean ± SEM ns indicates not significant. *P < .05 (t test or ANOVA). **P < .01 (t test or ANOVA). ***P < .001 (t test or ANOVA). (J) Analysis of immune tolerance induction in mice injected with 2 × 109 TU of ET.co-hFIXR388L.142T LV (n = 3). FIX-specific antibodies (mean ± SEM) were measured by ELISA at week 2 (w2) or week 4 (w4) after immunization with hFIX protein (ie, respectively, w8 and w10 after LV). FIX activity (mean ± SEM) was analyzed in parallel by chromogenic assay. Immunized PBS-injected hemophilia B mice (n = 3) were used as control.
Evaluating codon-optimized and hyperfunctional FIX transgenes by LV delivery in hemophilic mice. Mice were intravenously administered matched doses (in transducing units, as measured on 293T cells) of LVs expressing the indicated FIX transgene. FIX expression and clotting activity were measured by ELISA (A,C,E) and activated partial thromboplastin time (B,D,F), respectively, on plasma samples collected at the indicated times after LV administration. Vector copies per diploid genome (vector copy number [VCN]) were measured at the end of the experiments in liver genomic DNA. (A-B) A total of 5 × 108 TU (filled line) of ET.cFIX.142T (squares, n = 4; VCN 1.3 ± 0.1) or ET.co-cFIX.142T (triangles, n = 4; VCN 0.9 ± 0.2); 1 × 109 TU (dashed line) of ET.cFIX.142T (squares, n = 4; VCN 1.6 ± 0.3) or ET.co-cFIX.142T (triangles, n = 4; VCN 1.4 ± 0.4). (C-D) A total of 7 × 108 TU of ET.cFIX.142T (squares, n = 4; VCN 1.1 ± 0.2) or ET.cFIXR338L.142T (diamonds, n = 6; VCN 1.3 ± 0.3) or ET.co-cFIXR338L.142T (triangles, n = 7; VCN 2.2 ± 0.2). (E-F) A total of 2.5 × 108 TU (black line, n = 3; VCN 1.4 ± 0.2) or 1.25 × 108 TU (gray line, n = 3; VCN 0.6 ± 0.1) of ET.co-cFIXR338L.142T. Data are mean ± SEM. *pGLOBAL < .05 (nonparametric combination statistics). ***pGLOBAL < .001 (nonparametric combination statistics). (G) Tail-clipping assay on hemophilia B mice treated with 2.5 × 108 TU of ET.co-cFIX.142T (n = 3) or ET.co-cFIXR338L (n = 3) as indicated. Blood loss (mean ± SEM) was determined by measuring the absorbance at 575 nm of hemoglobin content in the saline solution in which the tail was placed (black bars, left axis); cFIX activity (white bars, right axis). WT (n = 5) and untreated hemophilia B (HemoB) mice (n = 5) were used as controls. (H-I) FIX expression and clotting activity were measured by ELISA and chromogenic FIX assay, respectively, on plasma samples collected at the indicated times after 109 TU (n = 4) or 2 × 109 TU (n = 3) of ET.co-hFIXR338L.142T LV administration as indicated. Data are mean ± SEM ns indicates not significant. *P < .05 (t test or ANOVA). **P < .01 (t test or ANOVA). ***P < .001 (t test or ANOVA). (J) Analysis of immune tolerance induction in mice injected with 2 × 109 TU of ET.co-hFIXR388L.142T LV (n = 3). FIX-specific antibodies (mean ± SEM) were measured by ELISA at week 2 (w2) or week 4 (w4) after immunization with hFIX protein (ie, respectively, w8 and w10 after LV). FIX activity (mean ± SEM) was analyzed in parallel by chromogenic assay. Immunized PBS-injected hemophilia B mice (n = 3) were used as control.
We next generated WT or co-cFIX transgenes bearing a point mutation corresponding to the previously described hyperfunctional FIX-R338L mutant that was shown to increase its specific activity 5- to 10-fold.10 As shown in Figure 1C, hemophilia B mice treated with LV carrying the WT or hyperfunctional FIX-R338L (7 × 108 TU/mouse) expressed ∼ 8% of normal cFIX protein in the circulation, whereas mice treated with the hyperfunctional co-cFIX-R338L transgene reached ∼ 20% (consistent with the aforementioned 2- to 3-fold increased expression of co-cFIX). However, both the hyperfunctional cFIX-R338L and co-cFIX-R338L transgenes exhibited > 5-fold higher activity with respect to the protein levels, resulting in up to 125% of normal clotting activity for the co-cFIX-R338L transgene (Figure 1D). These data show that the hyperfunctional co-cFIX-R338L transgene provides a 15-fold gain in potency with respect to the WT sequence and is greater than what has been reported with other FIX mutants.14,15
To evaluate whether the co-cFIX-R338L transgene allows lowering LV doses to reach therapeutic activity, hemophilia B mice were injected with 1.25 or 2.5 × 108 TU/mouse. Treated mice expressed ∼ 0.7 and 3.4% of normal cFIX protein, respectively. However, their clotting activity was ∼ 6.4% and 19% of normal, with 6- to 9-fold hyperactivity with respect to protein level (Figure 1E-F). None of the mice treated with hyperfunctional transgenes developed inhibitors (not shown). In a tail-clipping assay, hemophilia B mice treated with low-dose co-cFIX-R338L LV lost significantly less blood than mice treated with a matched dose of co-cFIX LV and were indistinguishable from WT mice, indicating the superior performance of the R338L FIX in achieving hemostasis in vivo (Figure 1G).
We reproduced these findings using LVs encoding a human hyperfunctional co-hFIX-R338L, resulting in 5- to 7-fold increased activity over protein levels at 2 different LV doses and reconstituting supra-physiologic clotting activity in hemophilia B mice (up to > 400% at the highest dose; Figure 1H-I). These mice did not develop anti-hFIX antibodies, even after challenge with hFIX protein, indicating that gene therapy induced immune tolerance to the WT protein (Figure 1J).
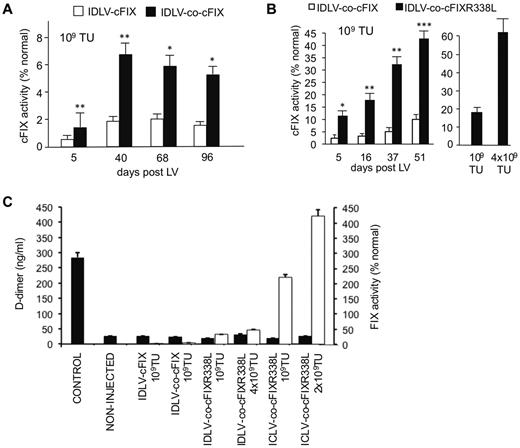
Although Integrase-Defective Lentiviral Vectors (IDLVs) exhibit a reduced genotoxic risk, hepatic transgene expression is less efficient compared with their integration-competent counterparts and tends to decline with time.9,16 We now show that using co-cFIX and hyperfunctional co-cFIX-R338L transgenes may offset some of the limitations of IDLVs, which now allow reconstituting FIX activity to fully therapeutic levels, exceeding 50% of normal FIX activity (Figure 2A-B). The extent of the increased expression and activity with these IDLVs was consistent with that observed with integrase-competent LVs.
Evaluating codon-optimized and hyperfunctional FIX transgenes by IDLV delivery in hemophilic mice. Mice were intravenously administered with the indicated doses (in transducing units), and clotting activity was measured by chromogenic FIX activity assays (A-C) on plasma samples collected at the indicated times after IDLV administration. (A) A total of 109 TU (n = 3) of ET.cFIX.142T IDLV or ET.co-cFIX.142T IDLV. (B) A total of 109 TU (n = 4) of ET.co-cFIX.142T IDLV or in a separate experiment 109 TU (n = 4) of ET.co-cFIXR388L.142T IDLV or 4 × 109 TU (n = 1) of ET.co-cFIXR338L.142T IDLV. (C) D-dimer levels (black bars) were determined by ELISA, and FIX activity (white bars) was analyzed by chromogenic assay in mice injected with different vector doses as indicated compared with noninjected control. The D-dimer positive control is shown. Data are mean ± SEM. *P < .05 (t test or ANOVA). **P < .01 (t test or ANOVA). ***P < .001 (t test or ANOVA).
Evaluating codon-optimized and hyperfunctional FIX transgenes by IDLV delivery in hemophilic mice. Mice were intravenously administered with the indicated doses (in transducing units), and clotting activity was measured by chromogenic FIX activity assays (A-C) on plasma samples collected at the indicated times after IDLV administration. (A) A total of 109 TU (n = 3) of ET.cFIX.142T IDLV or ET.co-cFIX.142T IDLV. (B) A total of 109 TU (n = 4) of ET.co-cFIX.142T IDLV or in a separate experiment 109 TU (n = 4) of ET.co-cFIXR388L.142T IDLV or 4 × 109 TU (n = 1) of ET.co-cFIXR338L.142T IDLV. (C) D-dimer levels (black bars) were determined by ELISA, and FIX activity (white bars) was analyzed by chromogenic assay in mice injected with different vector doses as indicated compared with noninjected control. The D-dimer positive control is shown. Data are mean ± SEM. *P < .05 (t test or ANOVA). **P < .01 (t test or ANOVA). ***P < .001 (t test or ANOVA).
To assess the possible risks associated with expression of hyperfunctional FIX, we determined D-dimer levels as a measure of fibrin degradation. D-dimers are not normally present in plasma, except when the coagulation system has been activated, as in the case of thrombosis.17 We did not detect a significant increase in D-dimer levels in treated mice, including those that expressed the highest levels of hyperfunctional FIX (Figure 2C), suggesting that thrombotic risk was not increased after gene therapy in these hemophilic mice, at least in the short term (1-2 months after injection). We also performed histopathologic evaluation of liver, spleen, kidney, heart, lungs, and brain of mice long-term reconstituted (10 months after LV) to supra-physiologic levels by co-cFIX-R338L LV (up to 125% of normal FIX activity), and we found no difference between mice treated with WT and hyperfunctional transgene (not shown).
Thrombosis risk is expected to be low at the levels tested, and further ad hoc studies in permissive thrombosis models are required to establish the long-term safety of delivering hyperfunctional FIX transgenes.18 Our data, together with the known impact of the R338L mutation on substrate interaction rather than zymogen activation,10 suggest that expressing limited amounts of hyperfunctional FIX to reach a threshold therapeutic level represents a viable and promising strategy to improve the efficacy, feasibility, and safety of hemophilia gene therapy.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. Sergi Sergi, S. Bartolaccini, and E. Samara-Kuko for technical assistance.
This work was supported by Telethon (TIGET D3; L.N.), EU FP7 (222878, PERSIST; L.N., M.C., and T.V.), and Bayer-Schering, FWO, GOA-EPIGEN (Free University of Brussels), EHA, and AFM (T.V. and M.C.).
A.C. conducted this study as partial fulfillment of his PhD in molecular and cellular biology at San Raffaele University, Milan, Italy.
Authorship
Contribution: A.C. and N.N. designed and performed experiments, analyzed data, and wrote the paper; P.D.V., M.D.M., and J.M. performed experiments and analyzed data; F.S. performed histopathologic evaluation; C.B. and C.D.S. performed statistical analysis; A.D. coordinated coagulation assays; and M.C., L.N., and T.V. designed experiments, coordinated the work, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi Naldini, San Raffaele Telethon Institute for Gene Therapy and “Vita Salute San Raffaele” University, Via Olgettina 58, 20132 Milano, Italy; e-mail: naldini.luigi@hsr.it; and Thierry VandenDriessche, Free University of Brussels, Laarbeeklaan 103, Brussels, Belgium 1090; e-mail: thierry.vandendriessche@vub.ac.be.
References
Author notes
A.C. and N.N. contributed equally to this study.
M.C., L.N., and T.V. share senior authorship.

![Figure 1. Evaluating codon-optimized and hyperfunctional FIX transgenes by LV delivery in hemophilic mice. Mice were intravenously administered matched doses (in transducing units, as measured on 293T cells) of LVs expressing the indicated FIX transgene. FIX expression and clotting activity were measured by ELISA (A,C,E) and activated partial thromboplastin time (B,D,F), respectively, on plasma samples collected at the indicated times after LV administration. Vector copies per diploid genome (vector copy number [VCN]) were measured at the end of the experiments in liver genomic DNA. (A-B) A total of 5 × 108 TU (filled line) of ET.cFIX.142T (squares, n = 4; VCN 1.3 ± 0.1) or ET.co-cFIX.142T (triangles, n = 4; VCN 0.9 ± 0.2); 1 × 109 TU (dashed line) of ET.cFIX.142T (squares, n = 4; VCN 1.6 ± 0.3) or ET.co-cFIX.142T (triangles, n = 4; VCN 1.4 ± 0.4). (C-D) A total of 7 × 108 TU of ET.cFIX.142T (squares, n = 4; VCN 1.1 ± 0.2) or ET.cFIXR338L.142T (diamonds, n = 6; VCN 1.3 ± 0.3) or ET.co-cFIXR338L.142T (triangles, n = 7; VCN 2.2 ± 0.2). (E-F) A total of 2.5 × 108 TU (black line, n = 3; VCN 1.4 ± 0.2) or 1.25 × 108 TU (gray line, n = 3; VCN 0.6 ± 0.1) of ET.co-cFIXR338L.142T. Data are mean ± SEM. *pGLOBAL < .05 (nonparametric combination statistics). ***pGLOBAL < .001 (nonparametric combination statistics). (G) Tail-clipping assay on hemophilia B mice treated with 2.5 × 108 TU of ET.co-cFIX.142T (n = 3) or ET.co-cFIXR338L (n = 3) as indicated. Blood loss (mean ± SEM) was determined by measuring the absorbance at 575 nm of hemoglobin content in the saline solution in which the tail was placed (black bars, left axis); cFIX activity (white bars, right axis). WT (n = 5) and untreated hemophilia B (HemoB) mice (n = 5) were used as controls. (H-I) FIX expression and clotting activity were measured by ELISA and chromogenic FIX assay, respectively, on plasma samples collected at the indicated times after 109 TU (n = 4) or 2 × 109 TU (n = 3) of ET.co-hFIXR338L.142T LV administration as indicated. Data are mean ± SEM ns indicates not significant. *P < .05 (t test or ANOVA). **P < .01 (t test or ANOVA). ***P < .001 (t test or ANOVA). (J) Analysis of immune tolerance induction in mice injected with 2 × 109 TU of ET.co-hFIXR388L.142T LV (n = 3). FIX-specific antibodies (mean ± SEM) were measured by ELISA at week 2 (w2) or week 4 (w4) after immunization with hFIX protein (ie, respectively, w8 and w10 after LV). FIX activity (mean ± SEM) was analyzed in parallel by chromogenic assay. Immunized PBS-injected hemophilia B mice (n = 3) were used as control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/23/10.1182_blood-2012-05-432591/4/m_zh89991299580001.jpeg?Expires=1767733969&Signature=Ke21xlP8-PqseRiSlBkxh5mpgRlKjQ5jffecJjBVcx7hI4ZiPYUK1HBRIesbZWTwFVfoKn~Wb3ToMRHIrtUmgZM4Rc2aYP6nK0v5BRRTx7SzCuEt0cwKrYOneb~kvIg3zCmljvEaYNo1BPBRrXmfIfIsNsX9WzNDkCRnZECzo5jrvK0-iYZ24pzj7m~~R7fvYgtQbJbxuJEYgGcutDp9DbTPKssmP2AsWlPiQ0hrUG7Ucr84PuSZtzgRAjz~6WxikkcRpp1hVNEa134hU1HPA2h39u89M-KAInBirD0RDy-Moy6hHGVUF0gAsl8e-55aNWSLoQfQLf3PHrsOcVTfrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal