Abstract
Abstract 983
Eculizumab, a monoclonal antibody that binds to the complement component C5, controls intravascular hemolysis in PNH patients and causes significant clinical improvement. However, the extent to which patients respond is variable in terms of hemoglobin levels, and some patients remain red blood cell (RBC) transfusion-dependent in spite of regular eculizumab treatment. We have hypothesized that genetic polymorphisms of complement-related genes might contribute to determining this variability of response to eculizumab. In order to test this hypothesis, we have analyzed polymorphic alleles of the Complement Receptor 1 (CR1) gene and of the Complement Component 3 (C3) gene. We have genotyped by standard methods (1) the HindIII restriction length polymorphism (RFLP) in the CR1 intron 27 (rs11118133 A>T) whose co-dominant alleles, H (common) and L (rare), are associated with high and low levels of CR1 expression on RBC, respectively; (2) the single nucleotide polymorphism rs2230199 C>G of the C3 gene, responsible for the allelic electrophoretic variants, S (slow, common allele) and F (fast, rare allele), that influence the complement alternative pathway activity.
We have studied 72 patients with hemolytic PNH treated with eculizumab for at least 6 months. Patients with clear evidences of bone marrow failure (platelets ≤ 50,000/μL, neutrophils ≤1000/μL) have not been included in this study because this condition may affect the clinical response independently of the control of hemolysis. In order for our criterion for response to be stringent and objective we used blood transfusion requirement: patients who received no RBC transfusion have been defined as Responders; patients who received any RBC transfusion have been defined as Poor Responders.
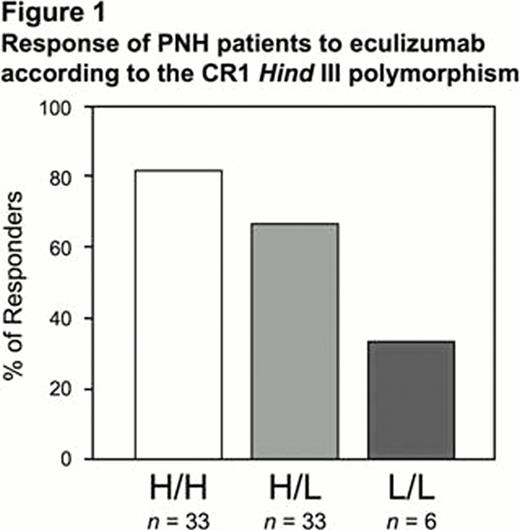
In this series of 72 hemolytic PNH patients on eculizumab the allelic frequency of the CR1 rare allele L was 0.31, and that of the C3 rare allele F was 0.19. We found no correlation between response and the C3 genotype (Chi square for trend: p=0.939). On the other hand the proportion of Poor Responders is significantly higher in patients who were heterozygotes (H/L) for the CR1 polymorphism, and even higher in those who were homozygous for the rare allele (L/L) of the CR1 HindIII RFLP polymorphism (Chi square for trend: p=0.016): see figure 1.
We do not know yet the precise mechanism whereby the CR1 genotype affects the response to eculizumab. CR1 enhances the decay of C3 and C5 convertase by binding to C3b and C4b and it plays a role in the clearance of immune complex and in phagocytosis. Thus, the variable levels of CR1 expression on RBC membrane associated with the HindIII RFLP polymorphism may affect the regulation of complement alternative pathway. Moreover, it is well established that in almost all PNH patients on eculizumab a significant fraction of GPI-negative RBCs are opsonized by C3 and therefore may undergo extravascular hemolysis. Thus, a possible explanation of our findings is that the lower levels of CR1 expression on RBCs associated with H/L and L/L genotypes are responsible for a more intense extravascular hemolysis in PNH patients on eculizumab.
In conclusion, our data show that the CR1 HindIII polymorphism may predict the poor response to eculizumab in PNH patients and provide additional insight in the mechanisms responsible for the variable clinical effectiveness of complement blockade.
Risitano:Alexion: Membership on an entity's Board of Directors or advisory committees, Research Funding. Peffault de Latour:Alexion: Consultancy. Iori:Alexion: invited speaker Other. Socie:Alexion: Consultancy. Luzzatto:Alexion: Consultancy. Notaro:Alexion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal