Abstract
Abstract 946
The emerging therapies directed at the plasma cell clone have dramatically increased overall survival (OS) for both myeloma and amyloidosis patient populations. As more treatment trials are done in patients (pts) with AL, benchmarks for outcomes among previously treated AL pts are required, especially for those pts who are not candidates for high-dose chemotherapy with peripheral blood stem cell transplantation (ASCT). Inadequate information is available about the number of non-transplant pts who go on to receive second line treatment for AL amyloidosis.
To ascertain the outcomes of pts who did not receive ASCT as primary therapy but who required a second line of therapy, we reviewed the experience of pts seen at Mayo Rochester between 1990 and 2010 according to an IRB approved retrospective review protocol. 1828 pts had their first Mayo visit for AL during this time period. Pts were excluded from this study for the following reasons: 571 had upfront transplant; 907 pts had received only one line of treatment for their amyloid; 91 had longstanding other associated malignancy [Waldenstrom's macroglobulinemia (8), lymphoma (15), CLL (6), multiple myeloma (62)]; and 93 had inadequate follow-up information. One-hundred and sixty-six pts received 2 or more lines of therapy for their AL amyloid and are the subject of these analyses. Statistical analyses were done using JMP statistical software (SAS, Carey, NC).
Of the 166 pts, the median age was 64 years (range 34, 84). 49% were male. Baseline organ involvement was as follows: cardiac 84/166 (50%); renal 118/166 (71); liver 23/166 (13.8%); peripheral nerves 19/166 (11.4%); and gastrointestinal 14/166(8.4%). Thirty-one percent (52/16) had both cardiac and renal involvement. Only 73 pts had cardiac biomarkers (NT pro BNP and Troponin T) done at baseline. Of these pts, 26% were stage 1, 49.3% were stage 2, and 24.6% were stage 3 according to original Mayo cardiac biomarker staging system.
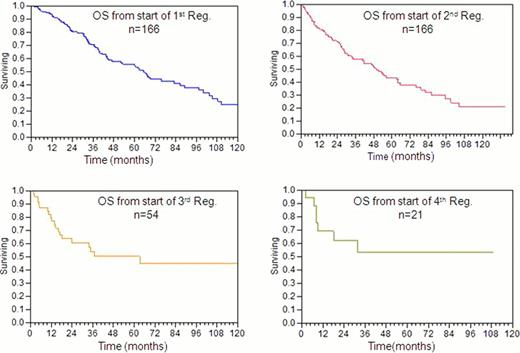
By inclusion criteria definition, all 166 pts received 1st and 2nd line therapies; 53 (32%) pts received 3rd line of treatment; 20 (12%) received 4th line of treatment; and 10 (6%) received 5th line of treatment. For first line therapy, the most common drugs given either singly or in combination were corticosteroid (147/166; 88.5 %), alkylator (99/166; 59.6%), IMID (34/166; 20.4 %), and bortezomib (19/166; 11.4%). The median time from diagnosis to 2nd line therapy was 10.3 months. Second line regimens received included: corticosteroid, 108/166 (65.1%); alkylator, 76/166 (45.8 %); IMID, 46/166 (27.7%); and bortezomib, 46/166 (27.8%). The median time from diagnosis to 3rd line therapy was 19.8 months. For the 53 pts who received 3rd line treatment, regimens included: corticosteroid, 41/53 (77.3%); IMIDs, 17/53 (32.1%); alkylator, 17/53 (32.1%); bortezomib, 12/53 (22.6%). The median time from diagnosis to 4th line therapy was 31.8 months. For the 20 pts receiving 4th line treatment, regimens included: corticosteroids, 15/20(75%); alkylator, 12/20 (60%), bortezomib, 7/20 (35%); and IMIDs, 4/20(20%). Eighty-three pts have died. The 1 year mortality of our study population was only 8%. The median follow-up of surviving pts was 47.6 months. Figure 1 demonstrates Kaplan-Meier estimates of overall survival (OS) from initiation of each successive therapy. The median OS from initiation of 1st, 2nd, and 3rd lines of treatment were 65, 49.5 and 36.7 months respectively. The median OS after the 4th line of treatment was not reached. The 4 year OS rates from initiation of 1st, 2nd, 3rd and 4th lines of therapy were 58%, 50.8 %, 50 % and 53.4% respectively.
Outcomes among relapsed or refractory AL pts are better than what one might expect. Multiple publications have demonstrated that the 1 year mortality of newly diagnosed AL is in the vicinity of 40%. Thereafter, the rate at which pts die dramatically decreases. Our study provides explicit data characterizing the fate of pts unfortunate enough to require additional therapy but fortunate enough to survive past the exceedingly high risk period of death that occurs within 6–12 months of diagnosis. These data provide useful information for benchmarking future trials for treatments of AL amyloidosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal