Abstract
Leg ulcers are a common and debilitating complication of sickle cell anemia and other hemolytic disorders. Despite many advances in the care of SCD, there is still a clinical need for a well-tolerated, safe and effective therapy. Sodium nitrite may function as a reservoir for local delivery of nitric oxide, whose vasodilating, angiogenic, and antimicrobicidal activities make it an attractive novel therapy for chronic wounds. We describe the safety data and preliminary efficacy data of topical sodium nitrite in adults with sickle cell anemia.
This is a phase 1 dose-escalation, single institution, study of topical sodium nitrite (ClinicalTrials.gov NCT01316796). Eligibility criteria: > 18 years old, have a leg ulcer of > 4 weeks duration, between 2.5 and 100 cm2 in size, and not acutely infected. Patients were screened with medical history, physical exam and laboratory tests. Sodium nitrite cream was applied twice a week for 4 weeks on one leg ulcer (“study ulcer”) in four cohorts of three subjects each with escalating concentrations in each cohort of 0.5., 1, 1.5 and 2%. Safety, tolerability and pharmacokinetic data of plasma nitrite and nitrate were obtained during the first 48 hours of drug exposure and then weekly for four weeks. Leg ulcer healing was a secondary endpoint and was assessed by calculating the change in surface area from visible light photographs of the study leg ulcer obtained at week 1, 3, and end of study, using a digital camera at a distance of 0.25 – 0.5 m from the ulcer. Borders were traced using ENVI software and ulcer size was calculated by converting the pixel area of the ulcer to cm2 using a calibrated square for reference. Pain at the ulcer site was assessed both with a Brief Pain Inventory and Visual Analog Pain Scale at predetermined time points.
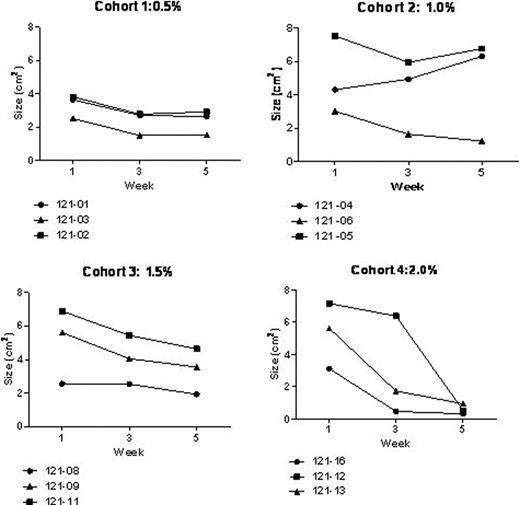
Sixteen subjects were screened and 12 enrolled. There were no serious adverse events associated with the drug. Possibly related, grade 1; adverse events occurred in 10 of the subjects, more frequently in the cohorts with the highest concentration, which included non-clinically significant decrease in diastolic blood pressure in two subjects of cohort 4. Adverse events resolved without clinical intervention. Methemoglobin levels did not exceed pre-established safety thresholds (max of 4.1 % in one subject in cohort 3). Tolerability was excellent, with short-lived stinging at the site of application reported by two subjects. Pharmacokinetics of plasma nitrite and nitrate indicated minimal systemic absorption of topically applied sodium nitrite (median plasma nitrite AUC: 0.311 (0.169–0.659) umol*h/L/umol nitrite dose), with high interpatient variability. There was no evidence of plasma nitrite and nitrate and methemoglobin accumulation during the 4- week study trial. All but one subject experienced a decrease in leg ulcer surface area, fig 1, (pretreatment 4.65 vs. post-treatment 2.78 cm2, p <.001) and improvement in pain scores (pretreatment 4.87 vs. 2.91, p=.024 ), which often preceded the decrease in size. Healing was most marked in cohort 4, the one treated with the highest strength of sodium nitrite cream, with 2 of 3 subjects experiencing complete closure of the ulcer.
On the basis of these safety, pharmacokinetic and tolerability data, and promising efficacy results, topical sodium nitrite warrants further clinical evaluation in patients with sickle cell disease or other hemolytic disorders and leg ulcers.
Changes in ulcer size during the trial by patient's cohort.
Changes in ulcer size during the trial by patient's cohort.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal