Abstract
Abstract 821
Sickle cell disease (SCD) is characterized by recurrent acute vaso-occlusive painful crisis frequently leading to SCD related complications such as acute chest syndrome, stroke, multi-organ failure and even sudden death. The complex pathophysiology of the vaso-occlusive painful crisis is mediated by activation of endothelial cells, adhesion of sickled erythrocytes and neutrophils, oxidative stress, coagulation activation and increased release of inflammatory mediators, resulting in ischemic organ damage. Recently, neutrophils have been demonstrated to form neutrophil extracellular traps (NETs) upon activation. Nucleosomes and histones exposed together with neutrophil proteases, such as elastase on these NETs have been shown to kill efficiently bacteria. NET formation has been shown to propagate coagulation in sepsis and in deep venous thrombosis. In addition, nucleosomes and histones exposed on NETs have been shown to be strongly cytotoxic to endothelial cells. Beside the exposure on NETs, nucleosomes can be actively released into the circulation from dead cells. Circulating nucleosomes detected in sepsis have been reported to correlate with severity of inflammation, organ dysfunction and mortality. However, no studies are available yet on the dynamics of nucleosomes and NETs in sickle cell patients suffering from painful crisis. The aim of this case-control study was to assess plasma levels of circulating nucleosomes and human neutrophil elastase–α1-antitrypsin (EA) complexes as measure of systemic neutrophil activation, in sickle cell patients during steady state and painful crisis.
Plasma levels of nucleosomes and EA as a measure of neutrophil activation were measured in 74 patients in asymptomatic state (49 HbSS/HbSβ0-thalassemia, and 25 HbSC/HbSβ+-thalassemia), 70 painful crises (53 HbSS/HbSβ°-thalassemia and 17 HbSC/HbSβ+-thalassemia) in 49 patients and in 24 HbAA healthy controls using Enzyme-Linked Immunosorbent Assay (ELISA).
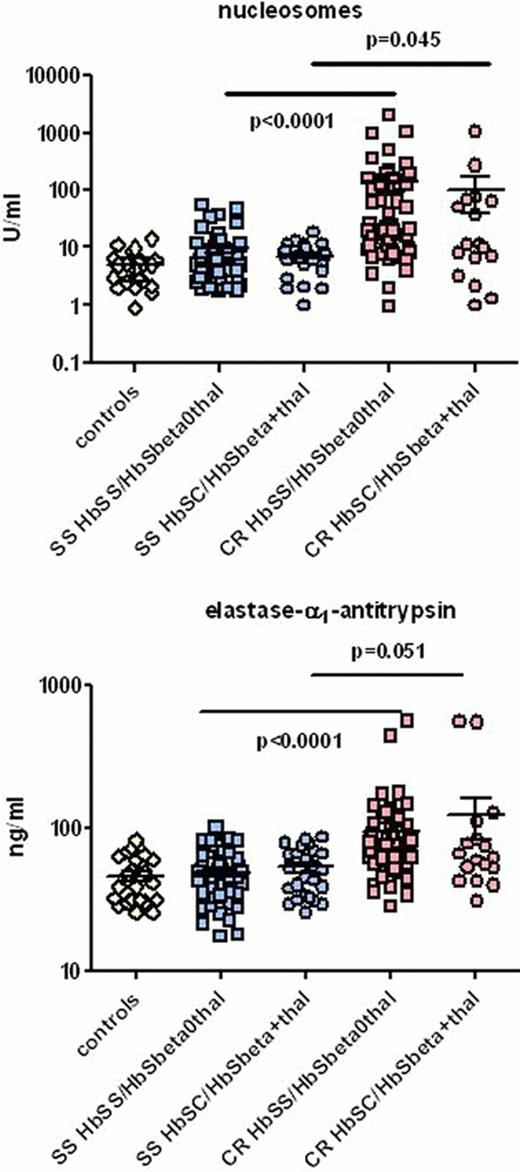
Plasma levels of nucleosomes in both HbSS/HbSβ°-thalassemia and HbSC/HbSβ+-thalassemia patients were significantly higher during painful crisis (median; interquartile range, 20.2; 8.9 – 129.0 U/ml, P < 0.0001 and 11.7; 5.1 – 67.7 U/ml, P = 0.045 respectively) as compared to patients in steady state (6.0; 3.0 – 9.8 U/ml and 7.1; 4.6 – 9.6 U/ml respectively). Nucleosomes levels in healthy controls were just above the detection limit of the assay (5.0; 5.0 – 6.5) U/ml).
Plasma levels of EA in HbSS/HbSβ°-thalassemia patients were significantly increased during painful crisis as compared to steady state (75.1; 56.5 – 102.4 vs. 45.7; 34.7 – 59.7 ng/ml, P < 0.0001). Also in HbSC/HbSβ+-thalassemia patients, EA levels were higher during painful crisis than in steady state, though the difference did not reach statistical significance (62.0; 48.0 – 96.7 vs. 50.2; 33.3 – 67.7, P = 0.051). Plasma levels of EA in healthy controls (39.9; 31.5 – 62.2 ng/ml) were comparable with those in steady state patients. In a paired analysis of 36 patients, included both during steady state and painful crisis, significant increments were observed during painful crisis in levels of both nucleosomes (from 5.0; 3.0 – 10.8 to 20.2; 6.8 – 94.3 U/ml, P < 0.0001) and EA (from 47.9; 36.0 – 67.6 to 70.6; 55.9 – 101.4 ng/ml, P < 0.0001), as compared to steady state. During painful crisis, EA levels were strongly correlated with levels of nucleosomes in both HbSS/HbSβ°-thalassemia (Spearman's rank (Sr)=0.55, P<0.0001) and HbSC/HbSβ+-thalassemia patients (Sr=0.90, P=<0.0001). In steady state the correlation was significant only in HbSC/HbSβ+-thalassemia patients (Sr=0.63, P=0.001) Four patients who developed an acute chest syndrome during painful crisis were among the patients with the highest nucleosome (359, 130, 128 and 100 U/ml) and EA levels (121, 87, 92 and 64 ng/ml respectively).
Sickle cell painful crisis is associated with increased levels of nucleosome and stronger neutrophil activation. This might point to a crucial role of NET formation in the pathogenesis of painful crisis.
Nucleosomes (upper panel) and elastase–α1-antitrypsin (EA) (lower panel) in healthy controls, HbSS/HbSβ°-thalassemia and HbSC/HbSβ+-thalassemia patients in steady state (SS) and during painful crisis (CR)
Nucleosomes (upper panel) and elastase–α1-antitrypsin (EA) (lower panel) in healthy controls, HbSS/HbSβ°-thalassemia and HbSC/HbSβ+-thalassemia patients in steady state (SS) and during painful crisis (CR)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal