Abstract
Abstract 795
Mogamulizumab (KW-0761) is a humanized anti-CCR4 antibody engineered to exert potent ADCC by defucosylation. In a phase I study for patients with CCR4-positive T-cell malignancies, once weekly administration for 4 weeks of mogamulizumab was well tolerated up to 1.0 mg/kg, and encouraging efficacy was observed (J Clin Oncol 2010;28:1591). In a subsequent phase II study in CCR4-positive relapsed adult T-cell leukemia-lymphoma (ATL) patients, mogamulizumab exhibited an overall response rate (ORR) of 50% (J Clin Oncol 2012;30:837), leading to its approval in Japan in 2012 for relapsed/refractory ATL. In addition, a phase I/IIa study for previously treated cutaneous T-cell lymphoma (CTCL) in the USA showed an ORR of 37% (14/38) (T-CELL LYMPHOMA FORUM 2012). Based on these findings, a phase II study of mogamulizumab for relapsed peripheral T-cell lymphoma (PTCL) and CTCL was conducted in Japan.
A multicenter phase II study of mogamulizumab monotherapy for patients with relapsed CCR4-positive PTCL and CTCL was conducted to evaluate efficacy, pharmacokinetic profile, and safety. The primary endpoint was ORR and secondary endpoints included progression-free survival (PFS) and overall survival (OS). At least 35 patients were needed to detect a lower limit of the 95% confidence interval (CI) exceeding the 5% threshold, with an expected ORR for mogamulizumab of 25% with 90% statistical power. Patients received intravenous infusions of mogamulizumab once per week for 8 weeks at a dose of 1.0 mg/kg. Responses were assessed after the 4th and 8th infusions of mogamulizumab by an independent efficacy assessment committee. The histopathological subtypes of PTCL were confirmed by an independent pathology review committee according to the 2008 WHO classification. In addition, we examined blood T-cell subset distributions.
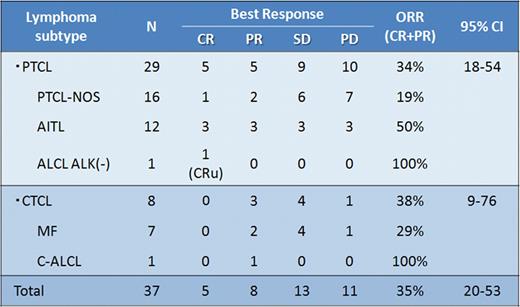
A total of 38 patients were enrolled, and 37 patients (male/female 23/14; median age 64 years, range 33–80) received mogamulizumab. One patient was withdrawn due to an infectious complication. Twenty-nine of the 37 assessable patients had PTCL [PTCL- not otherwise specified (NOS), n=16; angioimmunoblastic T-cell lymphoma (AITL), n=12; anaplastic large cell lymphoma (ALCL)-ALK negative, n=1] and 8 had CTCL [mycosis fungoides (MF), n=7; cutaneous ALCL, n=1]. Performance status at enrollment was 0 (n=24), 1 (n=12), and 2 (n=1). The median number of prior systemic chemotherapy regimens was 2 (range 1–6). Of the 37 patients, 25 completed the schedule of 8 planned infusions. Nine patients (24%) discontinued the treatment protocol due to progressive disease and 3 due to adverse events (AEs). The ORR in 37 patients was 35% (13/37, 95% CI, 20 to 53%) with 14% having a complete response (5/37) (Table 1). By PTCL subtype, the ORR was 34% (10/29) for PTCL (3/16 for PTCL-NOS, 6/12 for AITL, and 1/1 for ALCL-ALK negative) and 38% (3/8) for CTCL (2/7 for MF and 1/1 for cutaneous ALCL). AEs possibly, probably, or definitely related to mogamulizumab monotherapy were as follows. Lymphopenia of all grades and that of grades 3–4 were observed in 78% and 70% of the 37 patients, respectively. Leukopenia of all grades and that of grades 3–4 were observed in 43% and 14% of the 37 patients. For all grades and grades 3–4, neutropenia was observed in 35% and 16%, thrombocytopenia in 35% and 3%, ALT increases in 22% and 3%, and skin eruptions in 49% and 8% of patients, respectively. Infusion-related toxicities occurred in 22%, which were all within grade 2 or lower. Fourteen severe AEs were observed in 7 patients, including a grade 3 polymyositis in 1 and grade 2 cytomegalovirus retinitis in 2. All severe AEs were improved. No grade 5 AEs were observed. Pharmacokinetic analysis demonstrated that Cmax and trough (C168h) after the 8th infusion were 45.9 ± 9.3 and 29.0 ± 13.3 μg/mL, respectively. No anti-mogamulizumab antibody has been detected. Updated results of PFS, OS, and T-cell subset analysis are being analyzed for presentation.
Mogamulizumab monotherapy showed promising antitumor activity with acceptable toxicity profiles in patients with relapsed PTCL and CTCL, warranting further investigation.
Ishida:Kyowa Hakko Kirin Co., Ltd,: Honoraria, Research Funding, Speakers Bureau. Ogura:Kyowa Hakko Kirin Co., Ltd,: Consultancy. Suzumiya:Kyowa Hakko Kirin Co., Ltd,: Consultancy. Inagaki:Kyowa Hakko Kirin Co., Ltd,: Consultancy. Tamura:Kyowa Hakko Kirin Co., Ltd,: Consultancy. Akinaga:Kyowa Hakko Kirin Co., Ltd,: Employment. Tomonaga:Kyowa Hakko Kirin Co., Ltd,: Consultancy. Ueda:Kyowa Hakko Kirin Co., Ltd,: endowed chair Other.
T. Ishida and M. Ogura contributed equally to this work.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal