Abstract
Abstract 792
We previously reported the use of a sequential treatment (tx) program, R-CHOP-14 x4 followed by ICE x3, results in an 80% 5-y PFS (JCO 2010; 28 (23): 3754–3761) but interim (int) FDG-PET-4 (FDG-4) scan did not predict outcome. Patients (pts) with a positive (pos) FDG-4 underwent biopsy (bx), of which 85% were negative (neg). There was no difference in PFS for pts with pos FDG-4 and a neg bx vs. FDG-4 neg. In an attempt to reduce false pos FDG-4, we changed the induction tx as well as the timing of FDG-4. In addition, we prospectively evaluated 18F−fluorothymidine (FLT)-PET to determine its value for int evaluation. Proliferative index (PI) < or ≥80% risk adapted the consolidation therapy.
Eligible pts were <70 yrs with advanced stage DLBCL, FL grade 3B or primary mediastinal large B-cell lymphoma (PMBL). Pre-treatment evaluation included contrast-enhanced CT, FDG-PET and FLT-PET. Induction t× consisted of R-R-CHOP-14 x3 and CHOP-21 ×1. FDG-4 was performed 17–20 days after cycle 4 of therapy and a b× performed if pos. Consolidation was risk-adapted: FDG-4 neg or b× neg: ICE −3 for PI <80% and augmented RICE ×2 for PI ≥ 80%. Pts with a pos b× were treated with augmented RICE × 2 followed by HDT/ASCR. Use of FLT-PET was exploratory. In the first cohort, FLT-PET was repeated after cycle 1, and in the second cohort after cycle 2. Int FDG-4 was interpreted using the Deauville criteria (value of 4 or 5 was pos). FLT-PET was interpreted visually (neg FLT = uptake decreased to < blood pool and background). Delta SUV was calculated for both tracers to provide standardization.
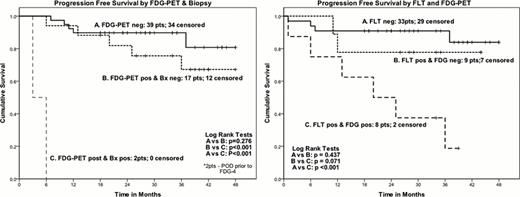
Sixty pts are evaluable; 50 underwent FLT-PET (10 pts could not be imaged due to lack of FLT availability or inability to schedule the test because of rapid tumor progression). Pt characteristics include: median age 54 (range 21–71), elevated LDH (82%); stage IV (75%); KPS <80 (25%) and age-adjusted (AA) IPI: low intermediate (LIR), HIR, and HR, 25%, 57% and 18% respectively. Pathology correlatives: PI ≥80 (35%); cell of origin (COO) via Hans model: GCB, ABC, PMBL, FL grade 3b: 42%, 33%, 22% and 3%, respectively. At median follow-up of 34 months for surviving pts, the PFS and OS are 79.3% and 85.6%, respectively. Pretreatment COO, AA-IPI, and PI were not predictive for PFS. The combination of altered induction therapy and timing of FDG-4 impacted on int evaluation. FDG-4 and int SUV max (>5) both predicted for PFS (p=.015 and .003, respectively). However, once again, pts with FDG-4 pos, bx neg evaluation had the same outcome as those with neg FDG-4 (p=.28) We combined the 2 FLT cohorts since the results were not significantly different. For the exploratory evaluation of FLT-PET, interpretation of FLT-PET-1/2 was based on delta SUV of >66% as a neg or favorable scan result. Thirty-three FLT-PET-1/2 scans were neg and 29 pts are progression-free (PF), including 8 pts with a pos FDG-4 (all had a negative bx and 7 are PF, hence 7 false pos FDG-4 scans). Among the 17 pts with pos FLT-PET-1/2, nine are PF. Nine FLT-PET-1/2 pos patients were FDG-4 neg and 7 are PF (hence improved response or false pos FLT). Lastly, pts with both pos FLT and FDG-4 did poorly; only 2 of 8 patients are PF.
These results confirm the excellent PFS of our sequential R-CHOP-14/ICE program. Altering the rituximab schedule, delaying int restaging by one week, as well as the use of FLT improved int evaluation. In this study, pts with a neg early FLT had an excellent outcome and likely no further imaging test is needed until the end of therapy for this cohort. In addition, pts with a neg FDG-4, despite a pos FLT, also did well with our induction/consolidation program. Future studies in these sub-groups may include reduced treatment strategies. Patients with dual tracer positive disease did poorly and the addition of novel therapy which may include kinase inhibitors with or without transplant is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal