Abstract
Abstract 79
Megaloblastic anemias are characterized by impaired DNA metabolism, often due to deficiencies in vitamin B12 or folate. Genes underlying hereditary forms of megaloblastic anemia not caused by vitamin B12 or folate deficiencies, however, remain largely unknown. Here we characterize a genetic deficiency in a patient with infantile-onset megaloblastic anemia, developmental delays, and a mitochondrial disorder of unknown etiology. Analysis of peripheral blood smears from the patient revealed hypersegmented neutrophils and erythroid macrocytes, classic features of megaloblastic anemias. The patient's vitamin B12 and folate levels are normal, eliminating their deficiency as potential causes of the disease.
Whole-exome sequencing of the proband cDNA identified a homozygous, single nucleotide deletion (c.231delC) in Sideroflexin-4 (SFXN4), a predicted mitochondrial multi-spanning transmembrane protein. We experimentally verified the mitochondrial localization of SFXN4 using a combination of western analyses on mitochondrial lysates and confocal fluorescence immunohistochemistry. Using trypsin-sensitivity assays on isolated mitoplasts, we further determined the submitochondrial localization of SFXN4 to the inner mitochondrial membrane.
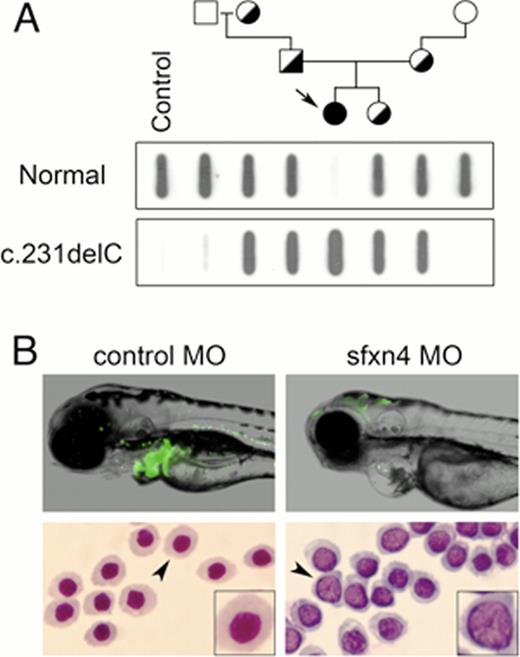
Bioinformatic analyses predict that the mutation introduces a frame shift and a premature stop codon (p.Pro78Leufs*25), resulting in a severely truncated polypeptide. To determine whether the mutant mRNA were expressed in vivo, we used qRT-PCR to assess the steady state level of SFXN4 mRNA in cultured fibroblasts from the proband. qRT-PCR revealed a 92% reduction in SFXN4 expression, consistent with nonsense-mediated decay of the mutant transcript. Genotyping of the index patient and 3 generations of her nuclear family using both Sanger sequencing and allele-specific oligonucleotide hybridization showed that the mutant allele is inherited in an autosomal recessive manner (Fig. A), the result of a presumed founder effect.
We used complementary zebrafish and human fibroblast systems to model the megaloblastic anemia and mitochondrial disease in the patient, respectively. Using splice-blocking antisense morpholino oligomers (MO) targeting sfxn4, we induced a loss-of-function phenotype in zebrafish embryos (hereafter, referred to as “morphants”). qRT-PCR confirmed the efficient knockdown of sfxn4, as morphants have <10% sfxn4 mRNA. Knockdown of sfxn4 in transgenic, Tg(globin LCR:eGFP) zebrafish showed a gross reduction in GFP+ erythrocytes and hemoglobinized cells stained by o-dianisidine (Fig. B, top), while quantification of the red cell population by flow cytometry showed a 60% reduction in the red cell mass. To characterize the anemia, we performed cytospins of flow-sorted erythroid cells from sfxn4 morphants and analyzed their morphology. Wright staining revealed that sfxn4 morphants have red cells with large nuclei containing non-condensed chromatin (Fig. B, bottom), consistent with the features of megaloblastic anemia observed in the index patient. Enumeration of the nuclear: cytoplasmic area ratios showed that red cells from sfxn4 morphants have a nearly 3-fold increase in the ratio of nuclear to cytoplasmic size.
We also investigated the mitochondrial disorder using patient fibroblasts, which showed a severe reduction in complex I (37%) and complex I+III (7%) activity. The over-expression of wild-type human SFXN4 in proband fibroblasts completely rescued the respiratory defect of complex I+III, while transfection of the mutant c.231delC SFXN4 construct failed to increase complex I+III activity. In a complementary strategy, the over-expression of wild-type SFXN4 cRNA from either zebrafish or human partially rescues the anemia in morphant embryos, validating their functional orthologous relationship.
In summary, a recessive loss-of-function mutation in SFXN4, a previously uncharacterized gene, causes the megaloblastic anemia and mitochondrial disorder described in the index patient. Genetic complementation studies in patient fibroblasts and sfxn4-silenced zebrafish morphants validate the pathogenicity of the mutation. Our findings: (1) demonstrate the requirement of SFXN4 for mitochondrial homeostasis and erythropoiesis, and (2) establish SFXN4 as a new candidate gene for mitochondriopathies and megaloblastic anemias.
No relevant conflicts of interest to declare.
J.D.C., G.J.H-S. and C.G. contributed equally to this study.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal