Abstract
Abstract 676
Although maintenance therapy has been shown to be of value in Acute Lymphoid Leukemia, its role in non M3 Acute Myeloid Leukemia (AML) has never been proven. Studies have been limited by issues related to efficacy as well as toxicity. The Eastern Cooperative Oncology Group (ECOG) trial, E2902, was a randomized phase III intergroup trial of the Farnesyl Transferase Inhibitor, R115777(tipifarnib), as maintenance therapy for AML. Patients(pts) with AML in remission after requiring salvage therapy or over age 60 in first remission were eligible. A confirmatory bone marrow exam prior to randomization was required to demonstrate a complete remission (CR) or morphologic remission(MR). Absolute neutrophil count >1000/mm3, platelet count >=50,000/mm3, normal renal, and hepatic function were required. Consolidation or post remission therapy prior to enrollment was allowed but not required. Pts who had received an allogeneic transplant in this remission were ineligible.
Pts were randomized to either receive tipifarnib twice daily (BID) or to observation. The main objective of the study was to compare disease free survival (DFS) in the two groups. The study was designed with 84% power to detect a 76% relative increase in the median DFS from 3.4 months to 6 months (mo). The final analysis was to take place when 111 events had taken place. Between August 2004 and December 2009, 144 pts were accrued to the study. The median age of the pts is 70 (range 28–86). The majority of pts enrolled on the study (67%) were in first CR. Seventy three pts were enrolled onto the treatment arm and 71 pts were enrolled onto the observation arm. When the study initially opened, tipifarnib was given at a dose of 400 mg BID with dose reductions and interruptions as per protocol for toxicity. Based on the first interim analysis, toxicity data, the starting dose was decreased to 300 mg BID. Significant hematologic toxicity was seen in patients at both 300 and 400 mg. The majority of patients experienced >=grade 3 neutropenia or thrombocytopenia. There was 1 episode of febrile neutropenia. Non-hematologic toxicities were minimal.
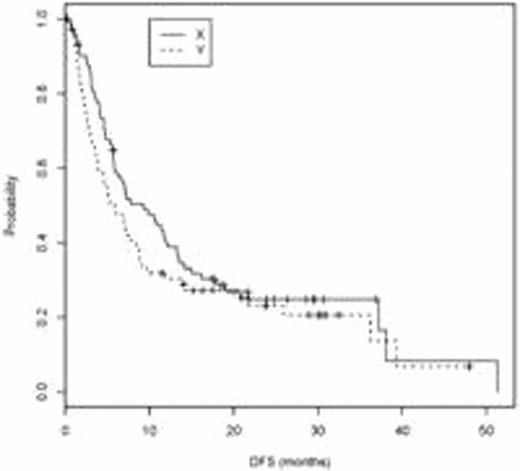
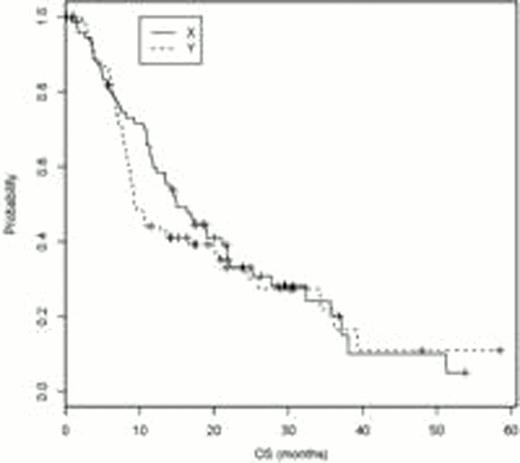
At the time of 110 events (90 relapses and 100 deaths), the final analysis of the study was conducted. The relapse rate was 60.3% on tipifarnib vs 64.8% for observation. Median DFS and 10 and 12 mo DFS and overall survival (OS) are documented in Table 1. Kaplan Meier curves for DFS and OS are presented. As the difference in median DFS is not considered significant (9.3 vs 5.8 mo, log rank p value 0.21), in July 2011, pts and physicians were informed of the lack of evidence of benefit so they could decide if they wished to continue drug. However an unplanned analysis of the data demonstrates a statistically significant improvement in OS at 10 months in pts who received tipifarnib. (70% vs 48%, p=0.05). While the benefit is subsequently lost, given a median survival measured in months, an increase in the number of pts alive and/or without disease at 10–12 mo is of value, particularly in the absence of noteworthy toxicity. Future studies are needed to better determine the role of maintenance therapy in pts with AML.
| . | Tipifarnib . | Observation . | . |
|---|---|---|---|
| Median DFS (months) | 9.3 (95% CI:5.7, 13.3) | 5.8 (95% CI: 3.7, 8.8) | p=0.21 |
| OS rate at 10 mo | 70% (95% CI: 58%, 79%) | 48% (95% CI: 36%, 60%) | p=0.05 |
| OS rate at 12 mo | 59% (95% CI: 47%, 70%) | 44% (95% CI: 32%, 55%) | p=0.07 |
| DFS rate at 10 mo | 47% (95% CI: 36%, 59%) | 32% (95% CI: 21%, 43%) | p=0.07 |
| DFS rate at 12 mo | 39% (95% CI: 28%, 50%) | 30% (95% CI: 20%, 41%) | p=0.26 |
| . | Tipifarnib . | Observation . | . |
|---|---|---|---|
| Median DFS (months) | 9.3 (95% CI:5.7, 13.3) | 5.8 (95% CI: 3.7, 8.8) | p=0.21 |
| OS rate at 10 mo | 70% (95% CI: 58%, 79%) | 48% (95% CI: 36%, 60%) | p=0.05 |
| OS rate at 12 mo | 59% (95% CI: 47%, 70%) | 44% (95% CI: 32%, 55%) | p=0.07 |
| DFS rate at 10 mo | 47% (95% CI: 36%, 59%) | 32% (95% CI: 21%, 43%) | p=0.07 |
| DFS rate at 12 mo | 39% (95% CI: 28%, 50%) | 30% (95% CI: 20%, 41%) | p=0.26 |
Larson:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy; Ariad: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal