Abstract
Abstract  595
595
Multiple myeloma is a malignancy of terminally differentiated plasma cells in which the malignant plasma cell clone usually produces a single abnormal unique monoclonal antibody with a constant isotype and light chain-restricted paraprotein. Recently, the occurrence of oligoclonal and monoclonal bands (OB/MB) not related to the original clone has been reported in patients with multiple myeloma who undergo autologous stem cell transplant (ASCT) and/or receive treatment with novel agents. Based on this data, the aim of our study was to assess the impact of monoclonal (MB) and oligoclonal bands (OB) occurrence on overall survival (OS) and progression-free survival (PFS) for MM patients undergoing single ASCT at Princess Margaret Hospital (PMH).
All consecutive patients with documented MM undergoing single ASCT at PMH from 01/00 to 12/07 were evaluated. Oligoclonal banding (OB) was defined as the development of two or more concurrent monoclonal-type bands on the serum electrophoretic pattern, with either a different heavy or light chain component from the original M-protein band at day+100 post-ASCT. A new monoclonal band (MB) was defined as a heavy and/or light chain immunoglobulin distinct from the initially diagnosed MM. All cases with OB/MB in our series fulfilled the criteria of secondary monoclonal gammopathy of undetermined significance (MGUS). Multivariate analysis was performed with the Cox proportional hazard model. All analyses were performed using the SPSS 13.0 software.
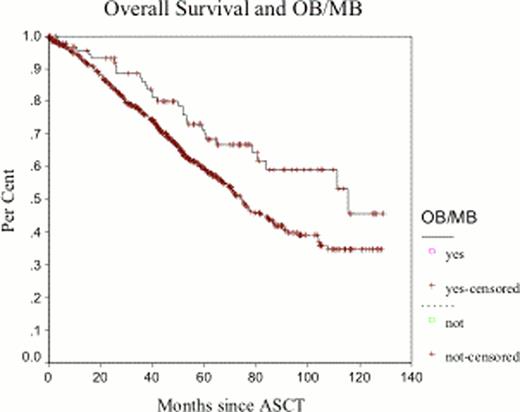
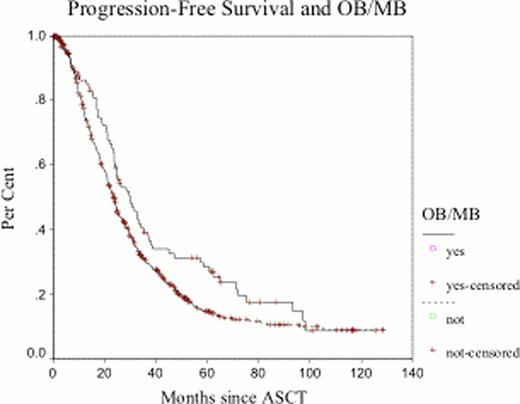
Between January 2000 and December 2007, 788 patients were identified. Clinical and laboratory characteristics are listed in Table 1 Ninety-six patients (12.1%) developed OB/MB at 3 months from ASCT: 32 patients (33.3%) had OB, and 64 patients (66.7%) had a new MB. The median duration of the OB/MB was 12 months (range 4–52 months). OB and MB emerged after ASCT in 14% (60/409) of patients receiving VAD, 7.0% of patients receiving bortezomib (6/86) and 8.6% of patients receiving thalidomide (6/69) containing regimens as induction therapy. Thirty-seven (38%) patients with subsequent development of an OB/MB had achieved ≥VGPR after induction and this rate improved to 79% (76/96) at day +100 post-ASCT. Patients who did not develop OB/MB had a ≥VGPR rate of 28% and 58% after induction and day+100 post-ASCT, representing a lower rate than patients with OB/MB (p=0.07 and 0.002, respectively). At the time of this analysis, 65 (67.7%) of the cohort patients who developed OB/MB are alive and 68 have already progressed (70.8%). Median overall survival for patients who did not develop OB/MB at day+100 post ASCT was 74.5 months compared to 115.5 months for those who developed OB/MB (p=0.0098). Multivariate analysis shows developing of OB/MB as an independent prognostic factor for OS and PFS (p=0.006 and 0.021, respectively). (Fig1a-b) The duration of the OB/MB did not affect OS and PFS.
In conclusion, OB/MB occurrence is an important prognostic factor in MM patients who undergo ASCT, the biological significance and its impact on clinical outcomes should be prospectively validated.
Clinical characteristics of patients with Multiple Myeloma Developing Oligoclonal and Monoclonal Bands after a Single ASCT.
| Clinical characteristic N=96 . | Median . | Range . | % . |
|---|---|---|---|
| Age (years) | 60 | 42–74 | |
| Male | 56.3% | ||
| Female | 43.8% | ||
| Hemoglobin (g/L) | 113 | 71–156 | |
| Creatinine (μmol/L) | 107 | 33–570 | |
| Calcium (μmol/L) | 2.28 | 1.88–2.73 | |
| LDH (IU/L) (N=90) | 184 | 66–353 | |
| B2-microglobulin ((μmol/L) (N=81) | 318 | 101–1930 | |
| Albumin (g/L) (N=65) | 38 | 21–47 | |
| Heavy chain at diagnosis | 39.5% | ||
| IgG | 44.2% | ||
| IgA | 1.2% | ||
| IgM | 0% | ||
| IgD | 9.3% | ||
| Biclonal | 5.8% | ||
| Not Detected | |||
| ASIP | 12.5% | ||
| IgG kappa | 30.2% | ||
| IgG lambda | 3.1% | ||
| IgA kappa | 4.2% | ||
| IgA lambda | 5.2% | ||
| IgM kappa | 11.5% | ||
| IgM lambda | 33.3% | ||
| Oligoclonal bands |
| Clinical characteristic N=96 . | Median . | Range . | % . |
|---|---|---|---|
| Age (years) | 60 | 42–74 | |
| Male | 56.3% | ||
| Female | 43.8% | ||
| Hemoglobin (g/L) | 113 | 71–156 | |
| Creatinine (μmol/L) | 107 | 33–570 | |
| Calcium (μmol/L) | 2.28 | 1.88–2.73 | |
| LDH (IU/L) (N=90) | 184 | 66–353 | |
| B2-microglobulin ((μmol/L) (N=81) | 318 | 101–1930 | |
| Albumin (g/L) (N=65) | 38 | 21–47 | |
| Heavy chain at diagnosis | 39.5% | ||
| IgG | 44.2% | ||
| IgA | 1.2% | ||
| IgM | 0% | ||
| IgD | 9.3% | ||
| Biclonal | 5.8% | ||
| Not Detected | |||
| ASIP | 12.5% | ||
| IgG kappa | 30.2% | ||
| IgG lambda | 3.1% | ||
| IgA kappa | 4.2% | ||
| IgA lambda | 5.2% | ||
| IgM kappa | 11.5% | ||
| IgM lambda | 33.3% | ||
| Oligoclonal bands |
ASIP: atypical serum immunofixation patterns.
Chen:Roche: Honoraria; Johnson & Johnson, Lundbeck, Celgene: Consultancy; Johnson & Johnson, Celgene, GlaxoSmithKline: Research Funding. Tiedemann:Janssen: Honoraria; Celgene: Honoraria. Kukreti:Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal