Abstract
Abstract 5070
In a recent phase III trial, azacitidine was demonstrated to significantly prolong OS compared with conventional care regimens in patients classified in intermediate-2 and high-risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System (IPSS) (Fenaux et al. 2009). This study used the French- American-British (FAB) classification for MDS and included approximately one third of patients with refractory anemia with excess blasts in transformation (RAEB-t; 20% to 30% bone marrow blasts). WHO criteria now define AML as ≥20% BM blasts. Using those criteria, RAEB-t is now considered as AML.
We conducted a retrospective analysis on patients who received azacitidine between August 2005 and November 2011 at our institution for MDS or AML. Patients were identified through the hospital database and individual charts were reviewed. The primary objective was to investigate the outcome of patients receiving azacitidine in a daily clinical practice in high risk MDS and AML patients and to evaluate its impact on overall survival (OS). Secondary objectives were hematological response rate and transfusion spare. Patients were included if they received at least one cycle of azacitidine. Disease status was defined by both the French American British (FAB) and the World Health Organization (WHO) classification systems, and risk was scored by the International Prognostic Scoring System (IPSS). All analyses were conducted using R statistical software. Descriptive statistics were used for baseline characteristics. Kaplan-Meier estimates were used to calculate overall survival (OS).
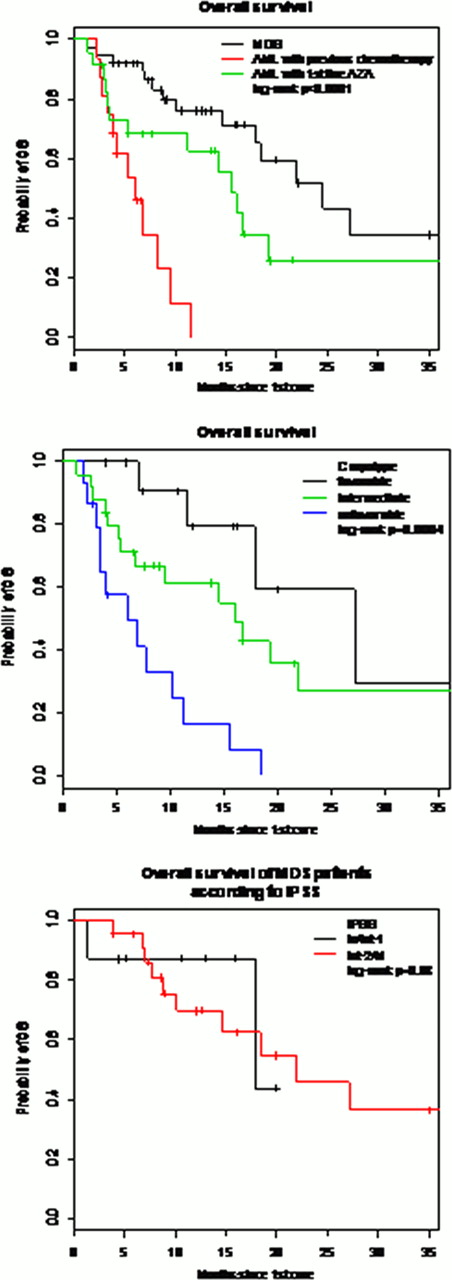
There were 79 patients, 51 (65%) males and 28 (35%) females with a median age of 70 years (32–85). The indication of azacitidine was the first line treatment use for MDS, mainly refractory anemia with excess blasts, in 40 (51%) patients (group1) and treatment for patients who had AML and transformed, in 39 (49%) patients (group2). (post chemotherapy: n=16, first line: n=23). Patient characteristics, prognostic factors according to FAB classification, ISPP risk and cytogenetics for both groups are shown in table1. The median number of azacitidine cycles in groups 1 and 2 was 8 (1–30) and 3 (1–29) respectively. Evaluation after 6 cycles showed 55% of responders in group 1 and 31% in group 2; the rest of patients have progressed. The median OS for the group 1 was 24. 5 months (17. 8-NR) while in group2; it was 15. 5 months (11. 2-NR) for patients who received AZA in first line and 6 months (3. 9-NR) for patients with previous chemotherapy. In terms of transfusions number, we did not find any significant spare in terms of both RBC and platelets transfusion in group1 while there was a significant spare of 33% of red blood cells transfusions (p=0. 05) and 42% of platelets transfusions only in group 2 (p=0. 04). The multivariate analysis studying the impact of different variables on OS showed: a worse OS in AML patients with previous chemotherapy (HR= 9. 84 [ 3. 56 – 27. 19 ], p< 0. 001), a worse OS in patients with unfavorable caryotype (HR= 7. 30 [ 2. 13 – 24. 98 ], p< 0. 001), and a better OS in female patients (HR= 0. 31 [ 0. 14 – 0. 68 ], p= 0. 003).
Our study confirmed results from previous prospective study in MDS patients while AML patients not receiving azacitidine in first line do not seem to benefit from this treatment. Cytogenetics remain a major factor impacting OS with no significant impact of IPSS.
Nicolini:Novartis, Bristol Myers-Squibb, Pfizer, ARIAD, and Teva: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal