Abstract
Abstract 5055
Classical chronic myeloproliferative neoplasms (MPNs) are amongst the best-characterized neoplasms associated with one of a set of specific somatic mutations, the most common of which is in the JAK2 gene. MPN is a relatively rare condition, and it could be surmised that it develops preferentially in people who have an increased tendency to somatic mutations, i. e. an increased somatic mutation rate (μ). Since measuring μ is rather labor-intensive, in this study we have measured instead the frequency of peripheral blood granulocytes that have inactivating mutations in a reporter gene, namely the gene PIG-A, a gene whose protein product is required for numerous glycosyl-phosphatidylinositol-anchored proteins to become surface bound. Since several such proteins are displayed by normal granulocytes, mutant cells can be numbered accurately by flow cytometry; and the frequency (ƒ) of such mutant cells may be a good surrogate of μ (Peruzzi et al. Mutation Research. 2010, 705:3).
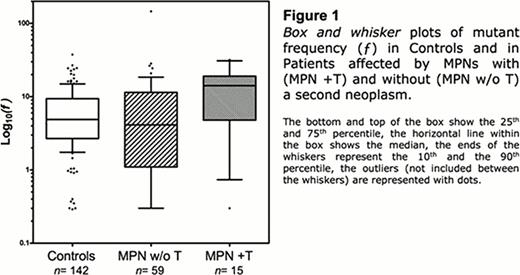
We have determined ƒ in 74 patients with MPN (including 29 with polycythemia vera (PV), 17 with essential thrombocythemia (ET), 16 with primary myelofibrosis (PMF) 12 with post-PV/post-ET (PPV/PET) MF. Overall the proportion of those with a JAK2(V617F) mutation was 90%. In a subset of 59 of these patients, who had “uncomplicated” MPNs, the median of ƒ was no different compared to 142 healthy controls (4. 74×10−6vs. 4. 87×10−6: Figure 1). However, in a subset of 15, consisting of patients who in addition to MPN had at least one primary malignancy (2 had lymphomas and 13 had one of several types of solid tumors), the median value of ƒ was 15. 22×10−6, i. e. significantly elevated (Mann Whitney test: MPNs vs. MPNs with a second neoplasm, p<0. 013; Healthy controls vs. MPNs with a second neoplasm, p<0. 005: Figure 1).
Clearly we cannot automatically extrapolate from our reporter gene, PIG-A, to the actual probability that another gene undergoes somatic mutation; besides, the rate of mutation of JAK2 is influenced by the local genomic context. With that proviso, our data suggest that JAK2 mutations are unlikely to result from an overall increased mutation rate. In keeping with this notion, we found no correlation between the value of ƒ and the allelic burden of JAK2(V617F) (R2= 0. 001): i. e., there is no evidence for genetic instability in MPNs (although, once there is a JAK2 mutation, this may produce a mutator phenotype). On the other hand, an intrinsically increased mutation rate does play a significant role in subjects with multiple neoplasms. This is not surprising: but, to the best of our knowledge, for patients who did not otherwise appear to be ‘cancer-prone’, direct evidence for this is provided here for the first time.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal