Abstract
Abstract 4968
In Multiple Myeloma immunophenotyping by multiparameter flow cytometry is a well-established method in clinical research to identify, characterize and enumerate neoplastic plasma cells. In addition, recent clinical studies demonstrated prognostic relevance of MRD detection by multiparameter flow analysis. The high applicability of the flow cytometric analysis compared to patient-specific RT-PCR holds the potential to become an important tool in MRD assessment.
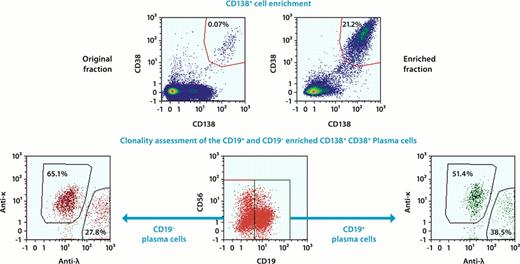
For the identification of plasma cells the detection of more than one antigen is required and for an unequivocal discrimination between normal and neoplastic plasma cells the combination with clonality assessment is mandatory. Here we established a combination of five antibodies for surface staining: CD138-PE, CD38-FITC, CD56-APC, CD19-PE-Vio770, CD45-APC-Vio770 and two antibodies for intracellular staining: Anti Igκ-PerCP, Anti Igλ-VioBlue. The combination of fluorochromes for this 7-color panel was optimized for high resolution of the target population.
To minimize variability among flow cytometric data, it is crucial to standardize the various procedures – in particular in clinical settings. Pre-defined antibody panels and simplified sample handling are major factors in the reduction of variability. Furthermore, in order to obtain maximal sensitivity processing of large cell numbers is required, which is a major obstacle for conventional flow cytometers.
Here we demonstrate that magnetic pre-enrichment of CD138+ in combination with a new protocol for in-column intracellular staining for κ/λ light chains of retained plasma cells allows handling of up 108 cells (fig. 1). Together with the optimized antibody panel this approach offers a unique and valuable tool for characterization of rare plasma cells and offers increased sensitivity during MRD assessment. The MACSQuant® Analyzer offers the possibility to combine magnetic pre-enrichment with intracellular staining and flow cytometric analysis. This minimizes manual handling steps and reduces cell loss due to omission of centrifugation steps usually required for intracellular staining procedures.
The approach of combined cell enrichment, in-column intracellular staining and usage of standardized antibody panels on the MACSQuant Analyzer provides an automated workflow, which is highly suitable for routine detection and characterization of rare plasma cells.
Kühbach:MiltenyiBiotec GmbH: Employment. Herber:Miltenyi Biotec GmbH: Employment. Koehler:Miltenyi Biotec GmbH: Employment. Schmitz:Miltenyi Biotec GmbH: Employment. Assenmacher:Miltenyi Biotec GmbH: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal