Abstract
Abstract 4898
Despite improved response rates with the use of novel agents, the vast majority of patients with multiple myeloma (MM) experience disease relapse and progression. This clinical pattern suggests the cells responsible for tumor regrowth persist following treatment, and we previously demonstrated that highly tumorigenic and self-renewing MM cells are relatively resistant to a wide range of anti-myeloma agents. These MM CSCs are present in the peripheral blood circulation and express memory B cell surface antigens (CD19 and CD27) and the stem cell marker aldehyde dehydrogenase (ALDH). We have also studied cellular mechanisms regulating self-renewal and found that MM CSCs express increased levels of telomerase activity (TA). In preclinical models, the novel telomerase inhibitor imetelstat has been found to inhibit CSCs from a wide range of tumor types, and we reported that imetelstat reduced TA in MM CSCs resulting in the loss of self-renewal and clonogenic growth potential.
In an open label phase II clinical trial initiated in February 2011, previously treated MM patients (pts) (n=6) with stable but detectable disease following treatment with an iMid or proteasome inhibitor received single agent imetelstat (7.5–9.4 mg/kg IV on days 1 and 8 every 21–28 days). Additional pts (n=4) receiving lenalidomide maintenance and whose disease was stable for at least three months have also been enrolled. Pts received imetelstat therapy until evidence of disease progression or toxicity. Median age is 62.5 years (range 53–68); median number of prior therapies is 1 (range 1–4) including 4 pts with prior autologous transplant and 1 pt with prior mini-allogeneic transplant; 8 pts with prior bortezomib use; 9 pts with prior iMiD use; and 4 pts with concurrent lenalidomide use with receipt of lenalidomide for a median of 11.38 months (range 4.51–19.21 months) prior to initiation of imetelstat. As of July 30, 2012, 6 pts remain on study. Four pts were discontinued from imetelstat therapy after receiving a median of 7 doses of imetelstat either due to disease progression (n=2) or hematologic toxicity (n=2). Cytopenias were the most frequently reported toxicity with 8 of 10 pts demonstrating grade 3–4 thrombocytopenia and neutropenia during cycle 2, often requiring dose reductions or holds in subsequent cycles.
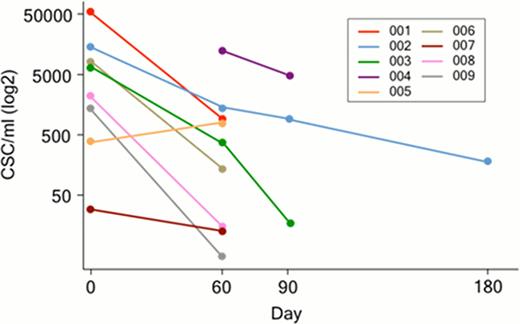
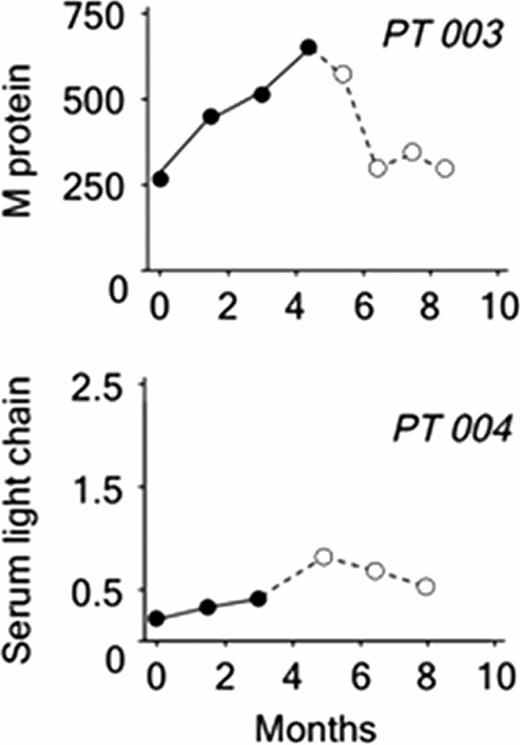
The frequency of circulating MM CSCs (CD19+CD27+ALDH+) was quantified by flow cytometry. Circulating MM CSCs could be detected prior to the initiation of imetelstat (mean 10.7 × 10e3 cells/ml, range 17–53 × 10e3) in 8 of the 9 pts who have completed at least 2 cycles of treatment with imetelstat. In the single remaining patient, circulating CSCs were assessed from Cycle 2 onwards. Over the course of treatment, the frequency of MM CSCs decreased significantly, on average 2 fold every 30 days (Fig 1), in 8 of the 9 patients studied despite no upgrades in clinical response as per IWG criteria. In two pts who received 4 and 6 cycles of single agent imetelstat respectively, standard responses detected as decreasing or plateaued serum M protein or light chain levels were sustained over 4 months following discontinuation (Fig 2). Moreover, these delayed responses occurred in the absence of any additional therapy.
These findings demonstrate that imetelstat rapidly decreases circulating MM CSCs. In addition, several patients experienced delayed, but sustained, clinical responses as measured by standard criteria. Therefore, imetelstat may have therapeutic implications for MM and other malignancies driven by CSCs.
Serial measurement of CSCs in patients (n=9) treated with imetelstat
Clinical response in Pts 003 and 004 based on changes in paraprotein level. Dashed line represents off study evaluation.
Clinical response in Pts 003 and 004 based on changes in paraprotein level. Dashed line represents off study evaluation.
Huff:Celgene: Scientific Advisory Board Other; Novartis: Consultancy. Jones:Cytomedix: Royalties, Royalties Patents & Royalties. Reddy:Geron Corp.: Employment. Nishimoto:Geron Corp: Employment. Stuart:Geron Corp: Consultancy, Employment; OncoMed Pharmaceuticals: Consultancy. Kelsey:Geron Corp: Employment. Matsui:Geron Corp.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal