Abstract
Abstract 4884
Ocaratuzumab, previously known as AME-133v, is a Fc- and Fab- engineered humanized anti-CD20 monoclonal antibody designed for optimized affinity to the CD20 antigen as well as to the FcγRIIIa (CD16a) receptor on effector cells. Compared to rituximab, ocaratuzumab has been optimized with a 13- to 20-fold increase in binding affinity to CD20 and improved binding to the low-affinity (FF and FV) polymorphisms of FcγRIIIa, which are thought to predict lower response rates and shorter duration of responses to rituximab. Ocaratuzumab also demonstrates 6-fold higher antibody-dependent cellular cytotoxicity compared to rituximab.
This abstract assesses the efficacy of a 100 mg/m2 dose of ocaratuzumab given once weekly for 4 weeks in previously treated follicular lymphoma patients with low affinity FcγRIIIa genotypes.
A total of 9 patients (6 in US and 3 in Japan) were treated with 100mg/m2 of ocaratuzumab given once weekly for 4 weeks as part of dose escalation studies. Response assessments to treatment were performed 9 and 21 weeks after the last infusion (Japanese study) or 9 weeks after the last infusion (US study) and subsequently every 3 months until disease progression, death, or until another therapy was initiated. Responses were assessed according to the 1999 NCI Working Group criteria.
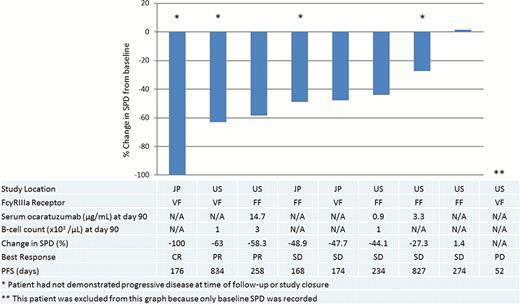
The 9 patients in this analysis included 5 males and 4 females, all of whom were F-carriers (4 FV and 5 FF); 1 patient was rituximab naïve. The median age of the patients at enrollment was 66 years (mean: 60 years, range: 37–75). Anti-tumor activity of ocaratuzumab was assessed as the decrease in the sum of products of the greatest diameters (SPD) from baseline. Tumor shrinkage was seen in 8 of the 9 patients (Figure 1). Three of the 9 patients achieved a response following ocaratuzumab (1 complete response and 2 partial responses). Five patients had stable disease and 1 patient had progressive disease.
The median progression-free survival (PFS) was 258 days (range: 52–834 days) which is similar to the median PFS of 50 patients who received a much higher dose of ocaratuzumab (375mg/m2 per week for 4 weeks) in a phase II trial. At the end of the study, 4 patients had still not progressed. Two of those patients had PFS exceeding 800 days.
The percent change in SPD from baseline following 4 weekly doses of 100mg/m2 of ocaratuzumab
The percent change in SPD from baseline following 4 weekly doses of 100mg/m2 of ocaratuzumab
Even when administered at approximately a quarter of the conventional dose of rituximab, ocaratuzumab demonstrated clinical activity in patients with relapsed follicular lymphoma. Administering ocaratuzumab at this low dose could substantially reduce the cost of therapy for patients with follicular lymphoma. Randomized trials will be needed to formally assess the clinical efficacy of low doses of ocaratuzumab in follicular lymphoma.
Le:Mentrik Biotech LLC: Employment. Smith:Genentech: Speakers Bureau. Link:Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Tobinai:Grant support: Eli Lilly, Zenyaku, Chugai, GSK, Biomedics Other. Du:Mentrik Biotech LLC: Employment. O'Reilly:Mentrik Biotech LLC: Employment. Jain:Mentrik Biotech LLC: CEO Other, Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal