Abstract
Abstract 4788
Thrombosis is a common complication of patients with malignancies. Patients with hematological malignancy have a 28 fold increase risk to develop venous thromboembolism (VTE). A population-based cohort was used to determine the incidence and risk factors associated with development of venous thromboembolism (VTE) among Californians diagnosed with acute leukemia between 1993 to 1999. Principal outcomes were deep vein thrombosis in the lower and upper extremities, pulmonary embolism, and mortality. Among 5,394 cases with acute myelogenous leukemia (AML), the 2-year cumulative incidence of VTE was 281 (5.2%). Sixty-four% of the VTE events occurred within 3 months of AML diagnosis. The induction of hypercoagulable state mechanisms is not fully understood to date. Multifactorial aspects such as physic immobility, chemotherapy adverse effects or the overexpression of several procoagulant substances (cytokines, cysteine protease and tissue factor) by cancer cells are often provoked. Several studies strongly suggest that microvesicles (MVs) harboring tissue factor activity may have a primary role in VTE. MVs are small membrane vesicles shed from normal and/or tumor cells following activation or apoptosis. MVs may present TF and negatively charged phospholipids (PL) such as phosphatidylserine on their membrane. These elements are thought to be implicated in the procoagulant activity (PCA).
The aim of this study was to assess the capacity of untreated acute promyelocytic leukemia cells to shed procoagulant MVs.
Acute promyelocytic leukemia (APL) cells lines (NB4 and HL-60) were cultured 48h in medium at 600,000 cells/mL. Cells and MVs were separated by filtrations (0.1–0.22–0.45–0.65μm). The PCA was assessed by thrombin generation assay. Alternatively, MVs were incubated with anti-TF antibodies (10μg/mL) or with annexin V (0,5μM to assess the contribution of TF and phospholipids to the PCA. Moreover HL-60 cells were incubated with HgCl2 which promote di-S bond formation (activation of TF).
NB4 cells and HL-60 cells can stimulate thrombin generation. HL60 cells reduced the lagtime 3.9-fold and increased the peak 1.6-fold in comparison to CTL and NB4 cells decreased the lagtime 10.9-fold and increased the peak 6.7-fold in comparison to CTL. No PCA was observed in HL-60 filtered with 0.65 μm membrane (no statistical difference in lagtime peak and ETP). By contrast, NB4 cells can support thrombin generation activity when filtered at different sizes. MVs of sizes <0.65 and <0.45 μm decreased the lagtime 4.2- and 3.8-fold, respectively and increased the peak of thrombin 4.6- and 4.1-fold, respectively. MVs of sizes lower than 0.22 and <0.1 μm reduced the lagtime 2.4- and 1.6-fold, respectively and increased the peak 2.3- and 1.4-fold, respectively. Thrombin generation activity of MVs of size <0.65 μm derived from NB4 cells wass abolished when anti-TF antibodies or annexin V were preincubated
Thrombin generation experiments. In control condition (CTL), normal pooled plasma (NPP) was spiked with PBS. NPP spiked with NB4 cells, and NB4 cells filtered through membranes with various sizes 0.1μm/0.22μm/0.45μm/0.65μm.
Thrombin generation experiments. In control condition (CTL), normal pooled plasma (NPP) was spiked with PBS. NPP spiked with NB4 cells, and NB4 cells filtered through membranes with various sizes 0.1μm/0.22μm/0.45μm/0.65μm.
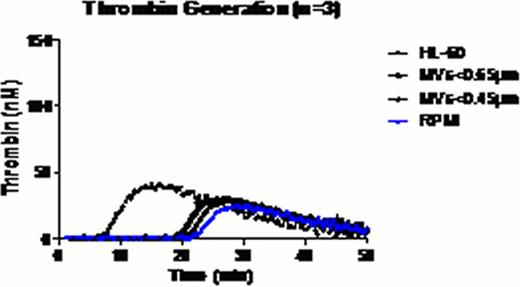
Thrombin generation experiments. In control condition (CTL), NPP was spiked with PBS. NPP spiked with HL-60 cells, and HL-60 cells filtered through membranes with various sizes 0.45μm/0.65μm.
Thrombin generation experiments. In control condition (CTL), NPP was spiked with PBS. NPP spiked with HL-60 cells, and HL-60 cells filtered through membranes with various sizes 0.45μm/0.65μm.
NB4 cells and HL-60 cells have different PCA. NB4 cells have a higher procoagulant activity and most of the PCA is linked to MVs of size under 0.45 μm. NB4 cells spontaneously release different sized MVs which can support thrombin generation. By using an anti-TF function-blocking antibody (HTF-1) and annexin V which binds phosphatidylserine, we confirmed that the PCA of MVs is related to the expression of active TF and PL. HL-60 cells have a weaker procoagulant activity because TF is mostly present in an inactive form (activation of TF by reduction agent such as HgCl2 increased the PCA of HL-60 cells of +/− 35%). Moreover HL-60 cells do not produce MVs<0.65 μm associated with PCA.
APL NB4 cells and HL-60 cells can stimulate thrombin generation. NB4 cells release MVs (of size <0.65 μm) whose procoagulant activity is mediated by TF and PL. These MVs could have a prognostic value for VTE in patient with APL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal