Abstract
Abstract 4759
Co-inheritance of Hb Rancho Mirage and Hb D-Punjab produces a clinically significant condition through increasing oxygen affinity that causes a compensatory erythrocytosis. We report a first case of co-inheritance of Rancho Mirage with Hb D-Punjab which differs in its clinical presentation from that described in the literature.
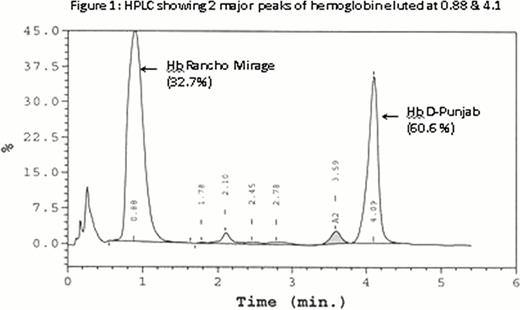
A 59-year-old Pakistani male was admitted to hospital because of chest tightness. The imaging studies showed no marked organomegaly or masses. Laboratory investigations revealed erythrocytosis with high hematocrit and borderline MCV and MCH. Arterial blood gas testing (ABG) showed low pO2 and JAK2 V617F mutation was not detected. Erythropoietin level was normal. Hemoglobin analysis through high performance liquid chromatography (HPLC) revealed two hemoglobin variants; Hb D-Punjab (32.5%) and the remaining Hb comprised of a fast moving variant. The variant was faster than Hb F expressing at a considerably high level. There was no detectable Hb A. (figure 1, HPLC shows both variants)
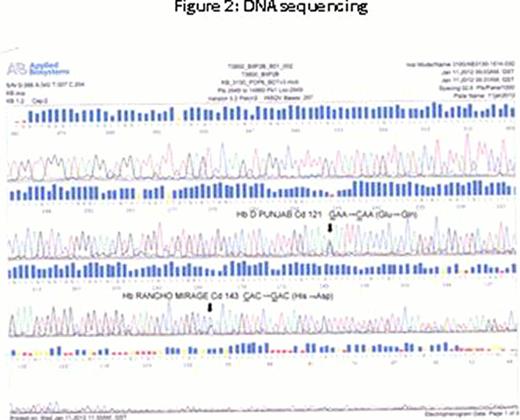
At the molecular level, the β globin gene was amplified and sequenced using specific primers. DNA analysis demonstrated the presence of two mutations in Exon 3. The first one was a rare mutation in βCd 143 (CAC→GAC) causing a substitution of His→Asp that was identified as Hb-Rancho Mirage. It is speculated that the replacement of the ‘His‘ residue with that of ‘Asp’ would unequivocally alter the oxygen binding characteristics of the heme pocket. The other mutation was the common variant defined as Hb D-Punjab βCd 121 (GAA→CAA). (figure 2, DNA sequencing shows both variants). The patient had undergone regular venesection to alleviate erythrocytosis.
The co-inheritance of Hb Rancho Mirage with Hb D-Punjab has never been reported previously. Both mutations exist in the β globin gene giving rise to concomitant beta chain variants. Carriers of Hb Rancho Mirage are anticipated with mild anemia. However, coexistence of both abnormal hemoglobins showed functional defects manifested as polycythemia, likely due to the disruption of the 2,3 DPG binding site which is actively involved in the allosteric equilibrium during oxygen transport.
The current case depicts the first occurrence of a double heterozygosity for two beta chain variants affecting exon 3 of the β globin gene with a relatively mild to moderate phenotype. Family studies are recommended to understand further the structure and function relationship of this rare hemoglobin variant in association with a more common Hb D- Punjab.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal