Abstract
Abstract 4735
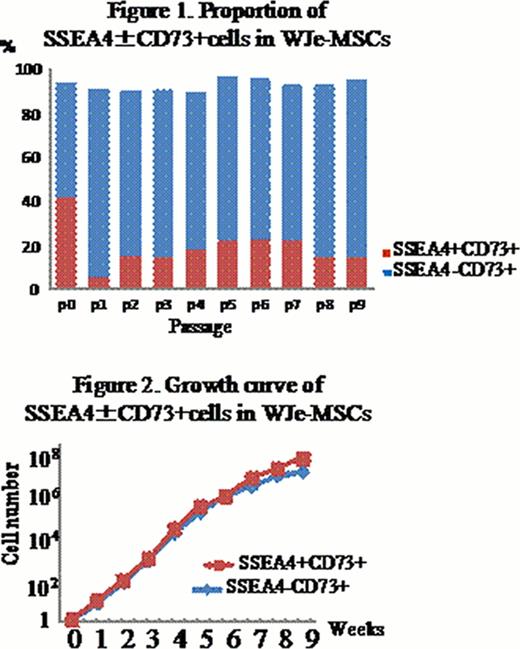
Wharton's jelly (WJ) of umbilical cord (UC) has been focused as a rich source of mesenchymal stem cells (MSCs), like bone marrow (BM) and adipose tissues. MSCs possess the capacity of self-renewal and multi-lineage differentiation. Despite the recent advantages in stem cell biology, surface marker antigen(s) that could represent the MSCs has not been identified. A stage-specific embryonic antigen 4 (SSEA4) was reported as a stem cell marker in BM-derived MSCs, and SSEA4+ cells have the advantage of growth and differentiation over the negative counterparts. To gain insight into the role of SSEA4 in WJ-derived MSCs (WJ-MSCs), we examined the incidence of SSEA4+ MSCs isolated from WJ according to the two distinct methods, and compared the biological characteristics between SSEA4+ and SSEA4− MSCs. After removal of artery and vein, WJ was chopped and plated on the plastic dishes by explants method (WJe-MSCs) or it was treated with collagenase and seeded onto the dishes (WJc-MSCs). After 3 to 4 weeks, the adherent cells were harvested and further analyzed. Expression of CD73 was significantly higher in WJe-MSCs (93.5%) than WJc-MSCs (55.1%) at the passage 0 (P0), while more than 90% of WJe-MSCs and WJc-MSCs were positive for CD73, CD105, CD90, HLA class I and negative for HLA Class II and CD34 after serial passages. RT-PCR analysis demonstrated embryonic stem (ES) cell markers including OCT4, NANOG, SOX2, KLF4 and REX1. We periodically monitored the expression of SSEA4 and SSEA3 vs CD73 in WJe-MSCs as well as WJc-MSCs during the culture periods (P0 to P9). WJe-MSCs at P0 had more SSEA4+ cells, compared with WJc-MSCs, significantly. Then, the number of SSEA4+WJe-MSCs declined during the passage (Figure 1.), and that of SSEA4+WJc-MSCs initially was low but turned to increase by P4, followed by declining. WJe-MSCs at P0 were divided into 3 fractions including SSEA4+CD73+ (41.7±17%), SSEA4−CD73+ (51.8±7%) and SSEA4+CD73− (1.8±2.3%) (n=5). In comparative analysis of WJc-MSCs at P0, the proportion of those fractions was 6.3±4.7%, 48.8±31.3% and 12.7±17.3%, respectively (n=5). Furthermore, the proportion of SSEA3+CD73+ cells at P0 were 8.5±7.6% in WJe-MSCs and 6.4±6.0% in WJc-MSCs, respectively (n=3). As a result, the explant method seemed superior to obtain MSCs efficiently. Next, we FACS-sorted SSEA4+CD73+ and SSEA4−CD73+ cells from the bulk culture of WJe-MSCs. Both types of cells showed similar growth rates (Fig. 2.) and could differentiate into adipocytes, confirmed by lipid deposition by Oil red O staining. Although osteogenic differentiation of WJe-MSCs was not likely in the conventional differentiation medium without bone morphogenetic protein 2 (BMP2) and variable among individual units, SSEA4+ cells tended to differentiate into osteocytes rather than SSEA4– cells, confirmed by alizarin red S staining. For hepatogenic differentiation, WJe-MSCs were plated on the collagen type I-coated dishes and induced by a three-step protocol including HGF and bFGF. SSEA4+, but not SSEA4−, cells could differentiate into hepatoocytes efficiently. Expression of albumin, a-fetoprotein and CK-19 was significantly higher in SSEA4+cells than SSEA4− cells. In conclusion, WJe-MSCs contained higher proportion of CD73+ and SSEA4+ cells from the beginning, compared to WJc-MSCs. Unlike the previously report for BM-derived MSCs, SSEA4+ WJ-MSCs could not show the growth advantage over SSEA4− cells, but had superior ability of differentiation into certain cell types.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal