Abstract
Abstract 4720
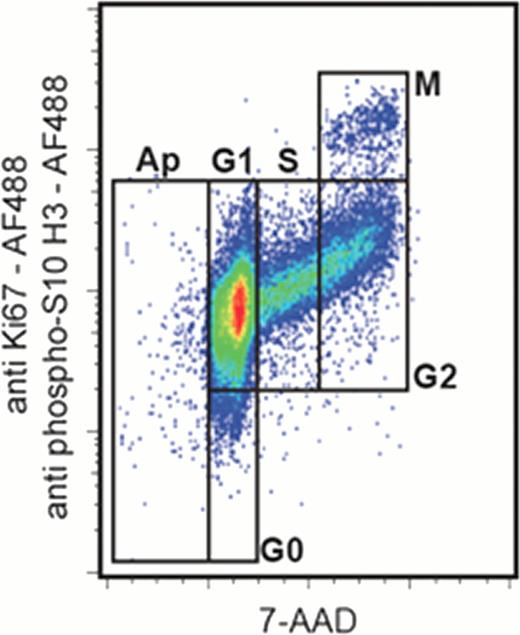
An optimal technology for cell cycle analysis would allow measuring concomitantly apoptosis, G0, G1, S, G2 and M phases in combination with cell surface phenotyping. We propose an easy method in flow cytometry allowing this discrimination in an only two-color fluorescent plot. It is based on the concomitant use of 7-amino-actinomycin D and antibodies anti-Ki67 and anti-phospho(Ser10)-histone H3 conjugated to Alexa Fluor.. 488 to discriminate G0 et M phases, respectively. The proposed method is particularly valuable in a clinical setting as verified by analyzing human leukemic cells from marrow samples or exposed to cell cycle modifiers.
The method was established using the human KG1a cell line and was applied to charcterize the cell cycle and apoptosis of fresh human marrow leukemic cells. The cells were permeabilized with 1 mL of ice cold ethanol (1 h, 4°C). Following two washes with PBS, 1% FBS and 0.25% Triton X-100 (PFT), the cells were stained in 200 μL of PFT for 30 min at room temperature in the dark with 1 μg 7-AAD (Sigma-Aldrich), 5 μL Alexa Fluor.. 488-conjugated anti-human Ki67 mAb (B56, Becton-Dickinson) and 3 μL Alexa Fluor.. 488-conjugated anti-phospho(ser10)-histone H3 polyclonal antibody (Cell Signaling Technology). A control tube was prepared with 1 μg 7-AAD and 5 μL of mouse IgG1 Alexa Fluor.. 488 (Becton-Dickinson). After 2 washes with PFT, the cells were stained with 10 μL of APC-Cy7-conjugated anti-CD45 (A20, Becton-Dickinson) followed by incubation for 20 min at 4°C. Cells were then washed twice with PBS, centrifuged for 5 min at 500 g and resuspended in 300 μL of PBS.
This flow cytometric method allows for a precise analysis of the impact on the cell cycle of various functional modulators. As an example, it was applied to analyze the pro-quiescent effects of contact with bone marrow MSCs as well as and the apoptosis induction and mitosis inhibition of the human KG1a leukemic cell line by camptothecin. The cell cycle characteristics of untreated KG1a cells were clearly quantified as follows: 0.4% sub-G1, 0.8% G0, 67.9% G1, 14.9% S, 14.2% G2 and 1.8% M phase. The contact with marrow MSCs during 72 h induced an increase in G0 phase and a decrease in M phase (5.3% and 0.4%, respectively). We verified the anti-proliferative and pro-apoptotic effects of 24 h exposure to camptothecin, which induced a decrease in S, G2 and M phases (6.1%, 6.2% and 0.4%, respectively) and an increase in sub-G1 phase (1.7%). Moreover, it is interesting to note that the staining protocol preserved the integrity of the plasma membrane and allows for the analysis of heterogeneous cell populations.
We document here the successful utilization of this method to discriminate concomitantly apoptosis and the cell cycle phases in a model of leukemic cells exposed to inducers of cell cycle perturbations. The value of this method to analyze heterogeneous populations was shown by discriminating the marrow cells from acute myeloid leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal