Abstract
Abstract 4532
Myeloproliferative Neoplasm (MPN)-Leukaemic Transformation (LT) uniformly carries a dismal prognosis. Effective therapy for such patients are currently lacking with no established evidence base to guide Allogeneic Haematopoietic Stem Cell Transplant (AHSCT) regimen. We report the outcome of a cohort of patients undergoing AHSCT at our 2 institutions over a 6-year period (2006–2012).
24 patients underwent AHSCT following diagnosis of MPN transformed to an accelerated phase (5–9% blasts on bone marrow (BM), n=9 & 10–19% BM blasts, n=2) or blastic phase (>20% blasts on peripheral blood or BM, n=13). Disease subtypes were: Polcythaemia Vera (PV, n=4), Essential Thrombocythaemia (ET, n=6), Primary Myelofibrosis (PMF, n=8), Myelodysplastic/Myeloproliferative neoplasm-Unclassified (MDS/MPN-U, n=6). Median age at diagnosis was 50 years (range 29–67) and median time to transformation was 50 months (range 0–271). Cytogenetics were abnormal at transformation in 11 patients (46%), with 6 (25%) demonstrating abnormalities of chromosomes 5, 7 or complex karyotype, and 5 displaying trisomy 8, whilst 1 had isolated chromosome 17p deletion. 13 patients harboured the JAK2V617F mutation.
Patients received a median of 3 (range, 1–5) lines of therapy for chronic and acute phase prior to AHSCT, of which 20 patients received intensive AML-type induction therapy. Disease status at time of AHSCT was complete remission (CR) in 13 cases, partial response (PR) in 7, and 4 patients had persisting AML. Conditioning regimes were Reduced Intensity with T-depletion (Alemtuzumab or Anti-Thymocyte globulin) in 23/24 cases (Fludarabine/Busulfan-based n=12, FLAMSA (Fludarabine, Ara-C and Amsacrine, followed by TBI/Cyclophosphamide or Busulfan) n=9, Fludarabine Cyclophosphamide TBI haploidentical protocol n=1, Fludarabine/Melphalan n=1). Median CD34 dose was 6.48 × 10∧6 cells/kg (range 1.17 {BMH} −10.71). Stem cell source was Peripheral Blood in all but one case, from unrelated (n=17) or related (n=7) donors. Median time to both neutrophil and platelet engraftment was 13 days (range 9–25 and 7–68 respectively); 2 patients including the haploidentical transplant had Primary Graft Failure (8%). The incidence of severe (grade 3&4) acute GVHD was 3/24 (12.5%) and 10 patients developed NIH-defined chronic GVHD (8 moderate, 1 severe). Day 100 Non-relapse mortality was 12.5%.
Patients underwent sequential chimerism monitoring. Median OS for the entire cohort was only 10 months with a median progression free survival (PFS) of only 6.5 months. 5 patients received therapeutic Donor Lymphocyte Infusion (tDLI) for relapse at a median dose of 1×10∧6 CD3+/kg. 2 patients received DLI alone for chronic phase relapse, of whom 1 achieved remission. 3 patients received chemotherapy + DLI and 1 achieved 2ndCR. At last follow-up, 11/24 patients were alive with median surviving patient follow-up of 25 months.
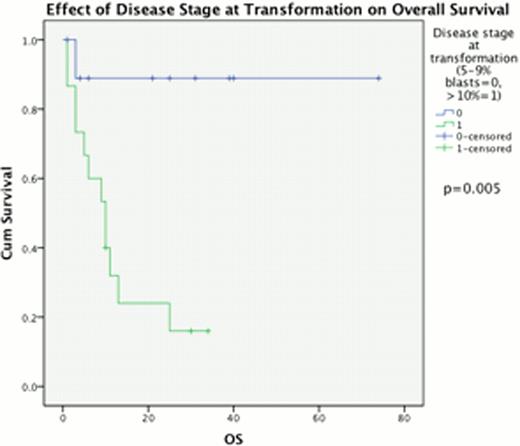
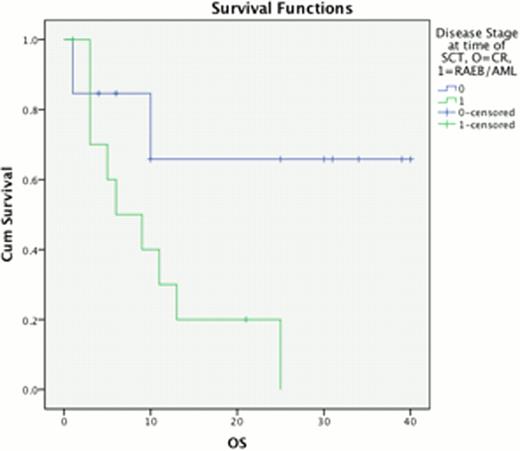
The percentage of BM blasts at progression from chronic phase had a highly significant impact upon outcome post AHSCT, median OS 23 vs. 10 months for 5–9% BM blasts compared to 310% BM blasts (p=0.011) & PFS 11 vs 6 months respectively (p=0.033, Fig 1). This effect was replicated when considering disease response immediately prior to AHSCT, with a median OS of 28 months for those in CR, compared to 10 months for those with excess blasts (p=0.017) and median PFS 11 vs 6 months, p=0.019 (Fig 2). Disease duration, subtype, Jak2 status and age at allograft did not significantly affect survival.
Of note for the 3 surviving patients with follow-up over 6 months, all received FLAMSA-RIC conditioning (n=9). 5 patients who received FLAMSA TBI; 2 died of treatment-related complications, and 2 with residual disease at time of AHSCT relapsed early. Of 4 patients who have received a hybrid FLAMSA-Busulfan regimen, 2 remain alive in CR and 2 achieved a relapse free period of 12 months. Interestingly, PFS for FLAMSA-Bu patients appears significantly improved compared to conventional RIC regimens (median PFS 12 vs. 6 months, p=0.035) on univariate analysis, although conclusions are limited by cohort size.
Further work into optimising transplantation regimens for accelerated and blastic phase MPN is warranted. Early use of FLAMSA-Busulfan hybrid protocol, before transformation to overt blastic phase, in conjunction with early weaning of immunosuppressive therapy and prophylactic DLI may improve the proportion of long-term survivors.
Harrison:Sanofi Aventis: Honoraria; Shire: Honoraria, Research Funding; YM Bioscience: Consultancy, Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal