Abstract
Abstract 4527
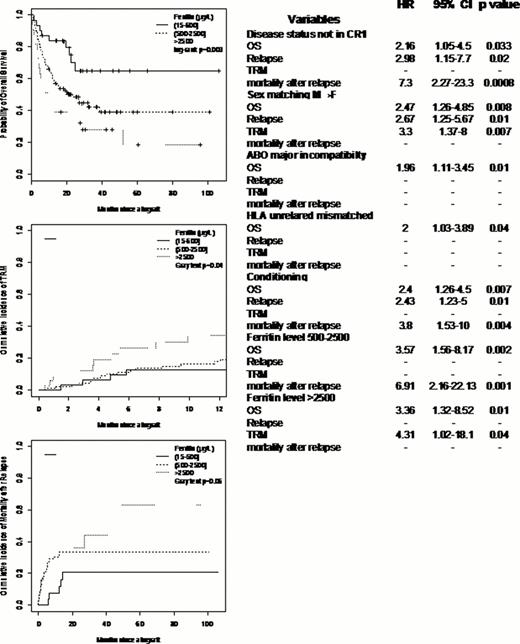
The main objective of this study was to determine the impact of serum ferritin level on the different allogeneic HSCT outcomes in adult patients with hematological disorders in addition to other disease and patient variables. We evaluated the implication of iron overload in the HSCT mortality causes and the potential role of iron chelation. We included 158 patients, 100 males and 58 females with a median age of 45 years (18–67) who underwent allo-HSCT between 2002 and 2010. There were 83 AML, 10 CML (3CP, 2AP, 5BC), 11 MDS (10 acute phase; 1 CMML), 7 myeloproliferative disorders, 19 MM, 9 NHL, 6 HDG, 5 aplastic anemia and 3 hemoglobinopathies. Sixty-seven (42%) patients were sex mismatched (F→M:37; M→F:30); for ABO compatibility, 61% were compatible, 18% had minor incompatibility and 21% had major incompatibility. For CMV status, 36% were sero-negative, 15% were D+R-, 18% were D-R+ and 31% were D+R+. Concerning the HSCT procedures, 60 patients (38%) received PBSC and 98 (62%) received BM from 97 (61%) HLA related donors [matched, n=76; mismatched, n=21], and 61 (39%) HLA unrelated donors [matched, n=36; mismatched, n=25] after MAC [n=64, (41%)] or RIC [n=94, (59%)]. At transplantation, 91 (58%) were in CR or CP [CR1: n=61 (67%); ≥CR2: n=30 (33%)] and the rest of patients were in less that CR or CP [n=67 (42%)]. The median serum ferritin level at HSCT was 1327 microg./l (26–14136); 31(20%) patients had a level 26–500, 33 (21%) had a level 500–2500, and 94 (59%) >2500. There were no significant correlation between the different ferritin levels, disease kind and status at HSCT. Twenty two patients received iron chelating agents (Exjade, n=19; Desferal, n=3). After allo-HSCT, the cumulative incidence of acute GVHD ≥ II at 3 months was 14% (11–16.5) with 10.5% (8–13) for grade III and 7% (5–9) for grade IV; the 1 year cumulative incidence of limited and extensive chronic GVHD were 4% (2–6) and 12.4% (9–16) respectively. After a median follow-up of 18 months (1–106), the median OS was 25 months (16-NR) with a 2 years probability of 50% (43–59). The median PFS was 13.5 months (9–25) with a 2 years probability of 43% (34–50). The cumulative incidence of relapse at 1 year was 31% (27–34), the TRM rate was 6.5% (4.5–8.5) and 20% (17–23.5) at 3 months and 1 year respectively. We performed a multivariate analysis taking into account the patient age, gender, diagnosis, disease status a allo-HSCT, matching variables (sex, ABO, HLA, CMV), conditioning/HSC source and the different serum ferritin levels; the different results are shown in table1. Interestingly, we found that ferritin level >500 has a significant impact on OS which is explained by a significant higher TRM for patients with level >2500 and a significant mortality after relapse in patients with level 500–2500 (figure).
The analysis on effect of iron chelating agents is ongoing and results will be communicated later.
Serum ferritin level has been previously shown to impact on allo-HSCT survival without a clear explanation of its implication and at which level; in our study, we demonstrated that this survival difference is caused in part by higher TRM and the other part by mortality after relapse.
In conclusion, we should give a better attention to iron overload, confer an efficient follow-up of this parameter and provide a treatment strategy after allo-HSCT especially when its level reaches 500μg/l at transplantation.
Nicolini:Novartis, Bristol Myers-Squibb, Pfizer, ARIAD, and Teva: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal