Abstract
Abstract 4511
The outcome for patients with primary refractory or refractory relapse of acute leukaemia, particularly those with disease present at the time of transplant, remains very poor even using full intensity conditioning schedules. With the aim of improving outcome, we have employed the use of pre-conditioning chemotherapy prior to the delivery of both reduced toxicity (RTC) and full intensity transplants during the subsequent period of cytopenia. Initial results indicate feasibility and efficacy in this poor risk disease. The bi-halogen purine analogue Clofarabine has significant activity as a single agent for the treatment of ALL and AML and is well tolerated, characteristics suggesting Clofarabine is an ideal agent to use in pre-conditioning.
We have treated 11 patients with high-risk, refractory acute leukaemia or advanced myelodysplasia (MDS) using Clofarabine pre-conditioning to reduce disease burden before full intensity or RTC allogeneic transplantation. Patient characteristics for the two groups are shown below (Table 1).
| Median age (range) . | Sex (%) . | Underlying disease (%) . | ||||
|---|---|---|---|---|---|---|
| M . | F . | Primary refractory AML . | Refractory Relapse of AML . | Refractory MDS (RAEB2) . | Primary refractory Biphenotypic acute leukaemia . | |
| 33 (17–58) | 4 (36) | 7 (64) | 2 (18) | 7 (64) | 1 (9) | 1 (9) |
| Median age (range) . | Sex (%) . | Underlying disease (%) . | ||||
|---|---|---|---|---|---|---|
| M . | F . | Primary refractory AML . | Refractory Relapse of AML . | Refractory MDS (RAEB2) . | Primary refractory Biphenotypic acute leukaemia . | |
| 33 (17–58) | 4 (36) | 7 (64) | 2 (18) | 7 (64) | 1 (9) | 1 (9) |
Clofarabine was administered as a single agent (40mg/m2/day for 5 days), five received RTC using Busulphan or Melphalan-containing transplant schedules; six full intensity total body irradiation (TBI) based conditioning. Indications for using Clofarabine included severe allergy to Cytarabine, heavy prior exposure to anthracycline or lack of response to other salvage agents. 10 of 11 patients had significant marrow involvement prior to administration of Clofarabine.
In this cohort we have not seen additional unexpected complications. One patient who received a RTC regimen, was treated for VOD on clinical suspicion but liver biopsy indicated grade 3–4 haemosiderosis only. Two patients who received TBI-based transplants suffered grade 3–4 mucositis. The extended period of pancytopenia compared with conventional transplant schedules was manageable, with no unexpected infectious complications. The median time to a neutrophil count of > 0.5 × 109/l was 18 days post stem cell infusion (range 12–33 days) and to a platelet count > 20 × 109/l was 13 days (range 11–32 days). All patients achieved ≥ 98% donor chimerism by day +30. 2 of 11 patients (18%) experienced grade 3–4 acute GvHD (1 cutaneous, 1 gut) which responded to additional immunosuppression. 9 of 10 patients with disease present at the time of Clofarabine achieved substantial bulk reduction or eradication of marrow leukaemic blasts following the Clofarabine. 5 of 11 patients died from disease relapse post allograft at 2–13 months post transplant, including the patient with Clofarabine-refractory disease. 6 patients are currently in CR at 1–77 months follow up.
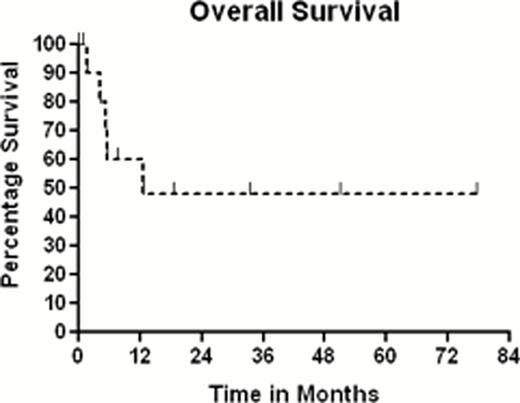
Overall survival for the 11 patients, using Kaplan-Meier analysis, is 48% with a median follow up of survivors of 26 months (range 1–77), shown below in figure 1:
The use of Clofarabine as a pre-conditioning agent prior to the administration of the transplant conditioning schedule is well tolerated with both full intensity and reduced toxicity protocols. This approach to allogeneic transplantation shows promise for the therapy of patients with high risk refractory acute leukaemia.
Richardson:Genzyme: Advisory board Other, Research Funding. Off Label Use: Clofarabine is licensed for the treatment of relapsed/refractory ALL in paediatric and young adult patients. This paper includes discussion of use in the treatment of AML/high risk MDS for which the drug has orphan drug status in the EU.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal